Atoms They are small particles of affair that make up everything in the universe. Everything you see is composed of atoms.
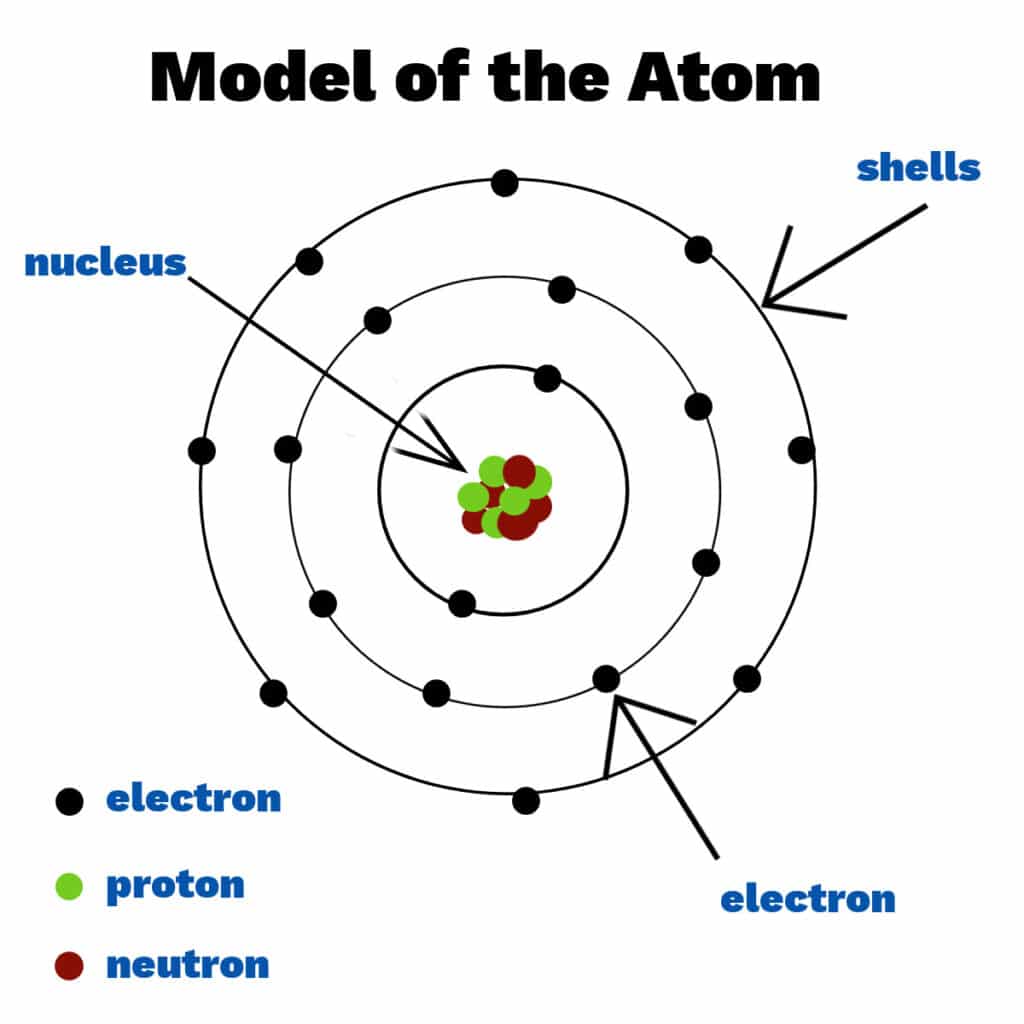
The scientists spent many years trying to understand the atom structurewith several models and theories refuted or improved along the way. Today we know that atoms consist of a nucleus that contains protons and neutrons, surrounded by shells (energy levels) of electrons. Electrons, protons and neutrons are known as Subatomical particles. Each housing contains a fixed number of electrons.
Atoms of different elements have different numbers of electrons, protons and neutrons.
Atomic structure theory
John Dalton
John Dalton is often known as the Father of atomic theory. Proposed a theory of atom in 1803.
John Dalton believed that:
All matter is made of atoms
The atoms were solid spheres – then refuted
The atoms within an element are the same. The atoms of different elements are not.
Atoms cannot be broken then refuted
Atoms are reorganized during a chemical reaction but do not miss. This is the Mass conservation law.
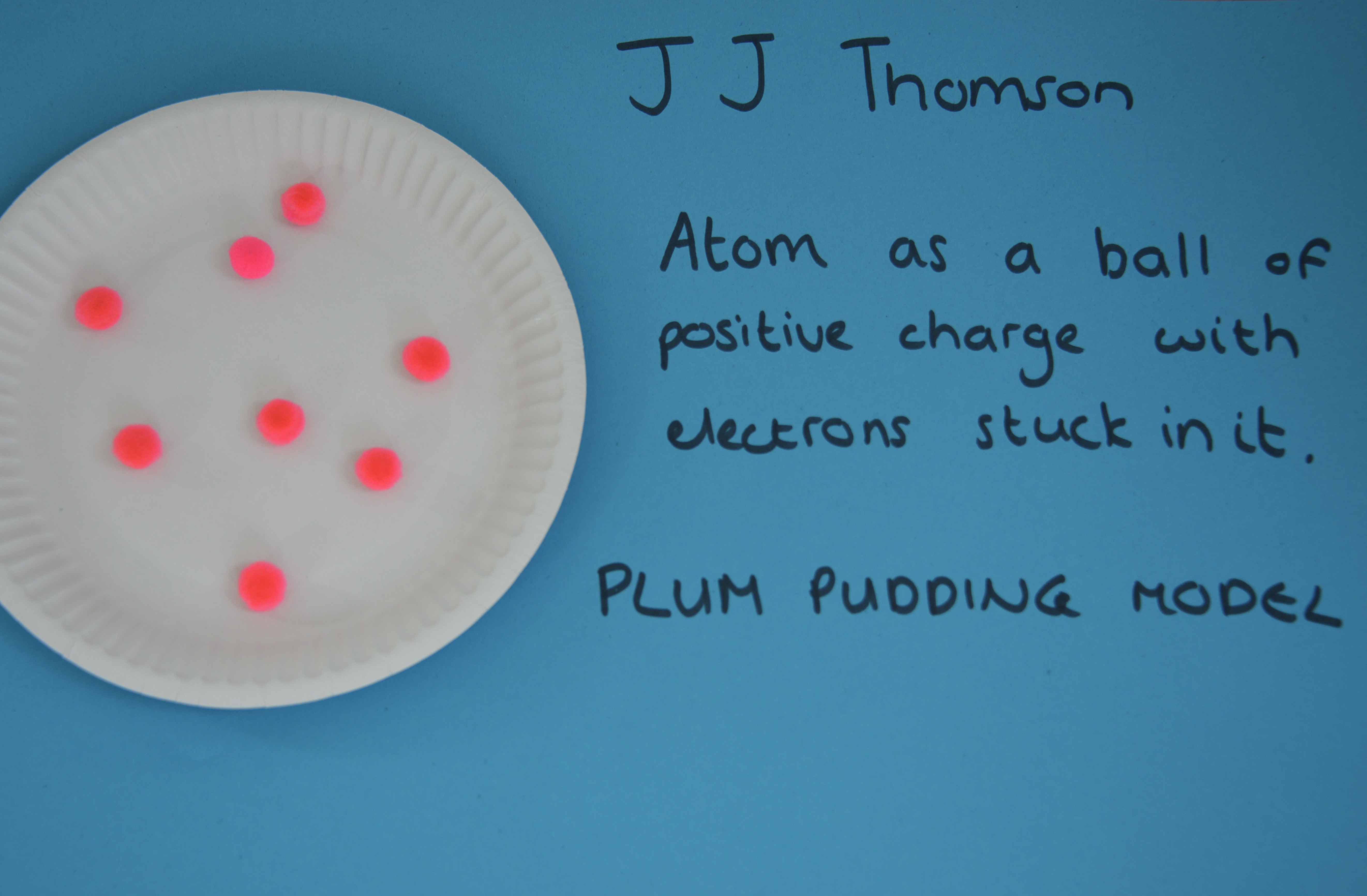
JJ Thomson
In 1897, JJ Thomson proposed that atoms were not solid spheres. His research showed that atoms must contain negatively charged particles (electrons). This theory is known as the Purum Buddine Model.
Ernest Rutherford
In 1909, Ernest Rutherford and two of his students made the now Infamo Gold aluminum experiment. They shot alpha particles positively loaded in a very thin gold sheet. If the plum pudding model were correct, the particles would pass through the gold sheet or deviate very slightly as the load was thought to spread through the atom. Gold was chosen as it can be very thin.
While most of the particles passed, some were diverted more than Rutherford was expected, and some were diverted backwards, demonstrating that the plum pudding model could not be correct. Rutherford developed a theory where the atom had a small core loaded positively in the center with a cloud of negative electrons that surround it.
The alpha particles fired to the gold sheet deviated back if they were close to the nucleus or passed through the empty space of the atom.

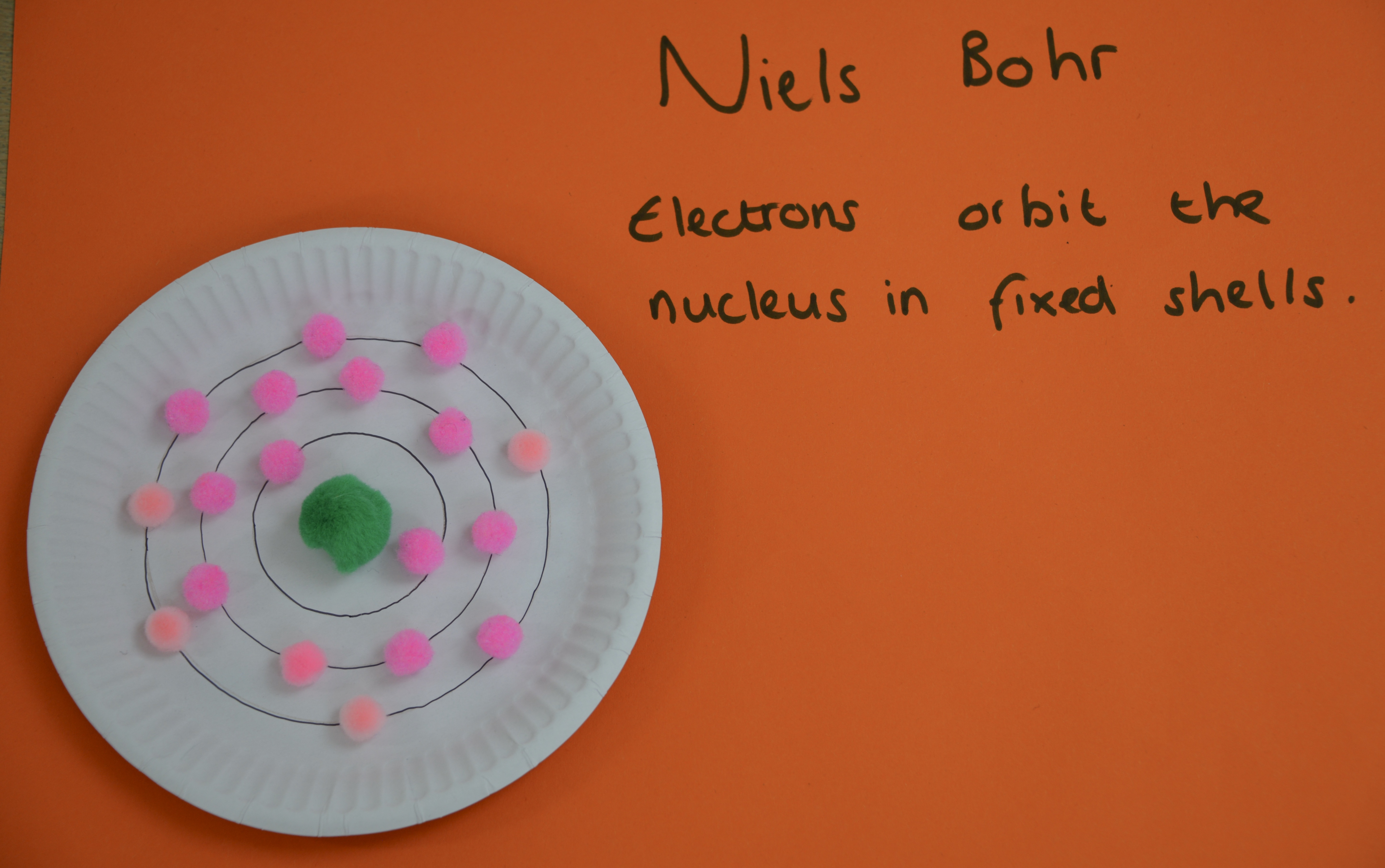
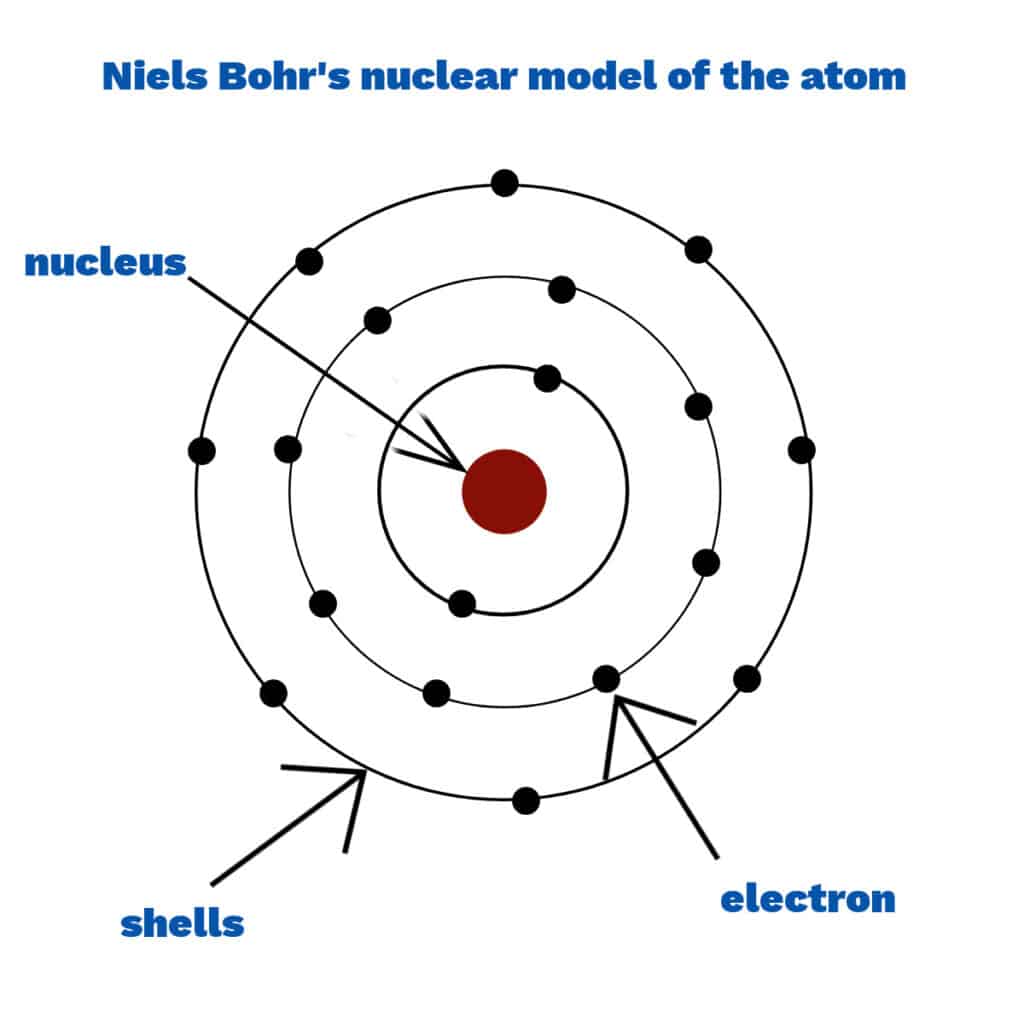
Niels Bohr
Niels Bohr developed an atom model with electrons arranged in fixed shells around the nucleus instead of a cloud. The scientists thought that the electrons in a cloud would be attracted to the nucleus, which makes the atom collapse.
James Chadwick
James Chadwick did experiments that show that atoms have neutral (neutrons) particles in their nucleus. James Chadwick’s model is very close to the modern nuclear model of the atom!

Learn more about the history of atom
Get more information about Ernest Rutherfordwho received a Nobel Prize for his work on atomic structure.
Discover how Ernest Schrödinger extended the model proposed by Bohr.

Last update on May 19, 2023 by Emma Vanstone
#history #atom