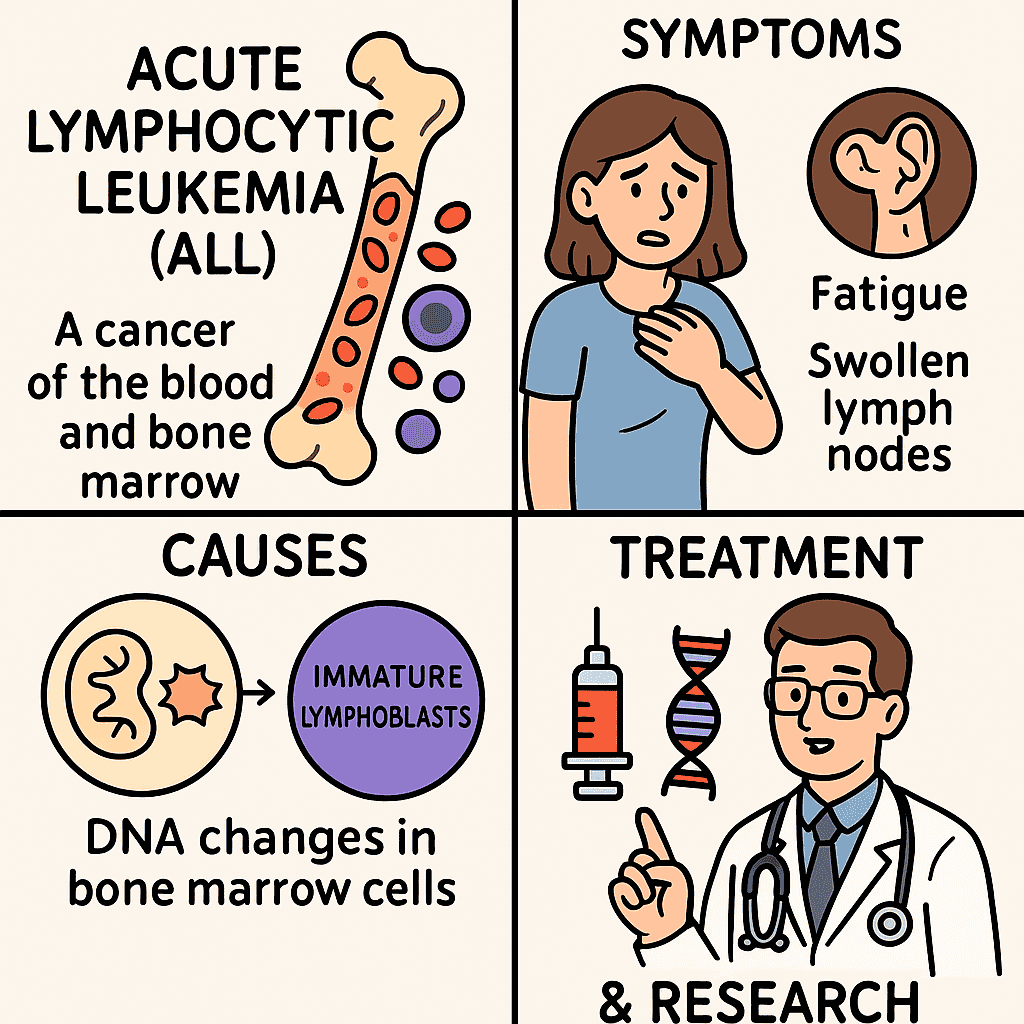Acute lymphocytic leukemia (all) is a type of blood cancer that begins in the bone marrow and grows rapidly if not. It mainly affects children, but it can also occur in adults, where results are often less favorable. In total, the body manufactures too many immature white blood cells called lymphoblasts, which move healthy cells and interrupt normal blood function. Over time, it can be extended to organs such as the brain, the liver and the spleen. Thanks to advances in research, treatments have improved, but there are challenges such as relapse and side effects of treatment. In this publication, we will explore what makes everything so complex.
Introduction
Acute lymphocytic leukemia (all) is an aggressive hematological malignancy characterized by the clonal proliferation of immature precursors, or lymphoblasts, in the bone marrow, blood and other organs. Although pediatric cancer is predominantly, with a maximum incidence between the ages of 2 and 5, everything also affects adults and has unique challenges among age groups.
For researchers and apprentices in biomedical sciences, understanding everyone’s molecular and cellular mechanisms is essential to contribute to continuous advances in diagnosis, risk stratification and treatment. This article offers an overview centered on everyone’s research, focusing on pathogenesis, classification and evolving therapeutic strategies.
Pathogenesis and cell origin
Everything originates from lymphoid progenitor cells in the bone marrow that acquire genetic and epigenetic alterations, interrupting normal differentiation and promoting unusual proliferation. These cells can commit to the Cell b either T cell The lineage, being B-there the predominant subtype in children and t-alalls more frequent in adolescents and young adults.
Molecular alterations generally imply:
-
Transcription factor deregulation (e.g, Pax5, IKZF1, ETV6)
-
Aberrant kinase signaling (e.g, Abl1, Jak2, FLT3)
-
Chromosomal translocations (e.g, T (12; 21)[ETV6-RUNX1], T (9; 22)[BCR-ABL1])
-
Abnormalities of the number of copies (for example, deletions in CDKN2A, IKZF1)
These mutations promote leukemogenesis by improving autramevation, stop apoptosis and block differentiation. In T-Lall, activation of the Notch route It is a central cancer event, observed in more than 50% of cases.
Epidemiology and Risk Factors
-
Incidence: ~ 4,000 new cases annually in the United States
-
Age distribution: Bimodal Peak: Children (2–5 years) and older adults (> 50 years)
-
Sex: Mild male predominance
-
Ethnicity: Greater incidence in white populations
Known risk factors
-
Genetic syndromes: Trisomy 21 (Down Syndrome), Bloom Syndrome, Telangiectasis of Ataxia, Fanconi anemia, Li-Franco’s syndrome
-
Exposure to high dose radiation
-
Previous chemotherapy (alkylating agents, topoisomerase inhibitors)
-
Hereditary mutations in DNA repair or cell cycle genes
Classification and molecular subtypes
Classification of all 2022 of all:
Leukemia/Lymphoma B-Linphoblastic (B-ALL):
Leukemia/T-linphoblastic lymphoma (T-ALL):
The molecular profile is now essential not only for diagnosis but to identify processable objectives (for example, ABL class mergers, activation of JAK-STAT, CRLF2 rearrangements).
Clinical presentation
Symptoms arise from medulla insufficiency, leukemic infiltration and metabolic deregulation:
-
Marrow failure: Anemia, thrombocytopenia, neutropenia → fatigue, bleeding, infection
-
Organ infiltration: Hepatosplanomegaly, lymphadenopathy, bone pain
-
SNC participation: Headache, vomiting, cranial nerve paralysis
-
Metabolic: Hyperuricemia, hypercalemia, tumor lysis syndrome
These non -specific symptoms often imitate viral infections or autoimmune conditions, which requires rapid hematological evaluation.
Diagnosis and work
Initial evaluation:
-
CBC and peripheral smear: Elevated WBC with lymphoblasts, anemia, thrombocytopenia
-
Bone marrow aspiration/biopsy: ≥20% lymphoblasts define all (according to whom)
-
Flow citometry: Immunophenotyped to classify the B-VS stage.
-
Cytogenetics/fish: Translocations detection (for example, T (9; 22), T (4; 11))
-
Molecular tests: RT-PCR or NGS to detect transcripts or fusion mutations
-
Lumbar puncture: To evaluate the participation of the CNC
-
MRD evaluation: Measured by flow cytometry or QPCR after induction
Minimum residual disease (MRD) is now a fundamental biomarker for the response to risk treatment and stratification.

General Treatment Description
1. Induction phase (~ 4 weeks)
Objective: Achieve complete remission (CR) eliminating> 99% of leukemic cells
Drugs: Vincristine, corticosteroids, L-asparaginase, ± anthracyclines
CNS prophylaxis begins through intrathecal metotrexate/citarabin
2. Consolidation/intensification phase
Objective: Eradicate residual disease and prevent systemic relapse/CNS
Includes dose of methotrexate, citarabine and additional intrathecal chemotherapy
3. Maintenance phase (2–3 years)
Objective: Delete late emerging clones
6-Daily-metapurine, weekly metretrexate, periodic vincristine/steroids
4. CNS prophylaxis
Universal, given a high risk of relapse of the CNS. It may involve intrathecal chemotherapy ± cranial irradiation (in selected high -risk cases)
Directed and immunotherapies
Tyrosine kinase inhibitors (tkis)
-
For PH+ all: Imatinib or Dasatinib with chemotherapy significantly improves the results
-
Newer tkis (for example, Ponatinib) used in mutant cases of T315i
CAR-T cell therapy
-
CAR-T directed by CD19 (eg
-
Main adverse effects: cytokine release syndrome (CRS), neurotoxicity syndrome associated with immune cells (ICans)
Biespecific cell commissioners (bites)
Conjugated with antibody-peer
-
Inotouzumab ozogamicin: Anti-cd22 ADC used in recurring/refractory B-ALL
-
Associated with veno-ratclusive disease, especially after transplantation
JAK-STAT road inhibition
Prognostic factors
Favorable:
-
Age of 1 to 10 years
-
WBC <50,000/µl
-
Hyperdiploidia
-
ETV6-RUNX1 fusion
-
Rapid MRD rappers
Unfavorable:
MRD negativity (<0.01%) is currently the most powerful predictor of long -term remission and survival.
Emerging challenges and addresses in research
-
Recurring/refractory everything It is still a great obstacle; Molecular profile guides Rescue strategies.
-
Lineage plasticity and escape antigen (e.g., CD19 Loss after car) has therapeutic challenges.
-
Clonal evolution Under selective pressure of therapy highlights the need for longitudinal genomic monitoring.
-
Improvement of results in adults and high -risk subgroups (for example, T-ALL, Hypodiploid B B-ALL) requires greater translational investigation.
-
Epigenetic deregulation (e.g, CREBBP, NSD2) and Metabolic vulnerabilities Offer possible new objectives.
-
Next generation immunotherapies (for example, trispecific antibodies, double objective cars) are in early clinical development.
Conclusion
Acute lymphocytic leukemia serves as a model disease to understand clonal evolution, directed therapy and immunosos oncology. Despite the significant improvements in pediatric survival rates, unsatisfied needs remain in adult populations and recurrent diseases. As research continues to dissect the genetic, epigenetic and micro -environmental drivers of all, future therapies will probably be customized more and more.
For medical research apprentices, everyone offers a rich platform for study: expansion of stem cell biology, immunotherapy, systems genomics and medication resistance. Continuous collaboration between doctors and scientists will be critical to advance the results for all patients affected by this aggressive leukemia.
#Acute #lymphocytic #leukemia #general #research #oriented #description
![[Botany • 2025] Thismia selangorensis (Thismiaceae) • A new mitriform species from the Thismia sect. Geomitra from Selangor, Malaysia](https://thenewshub.website/wp-content/uploads/2025/12/Thismia_selangorensis-novataxa_2025-Siti-Munirah_Siew-150x150.jpg)