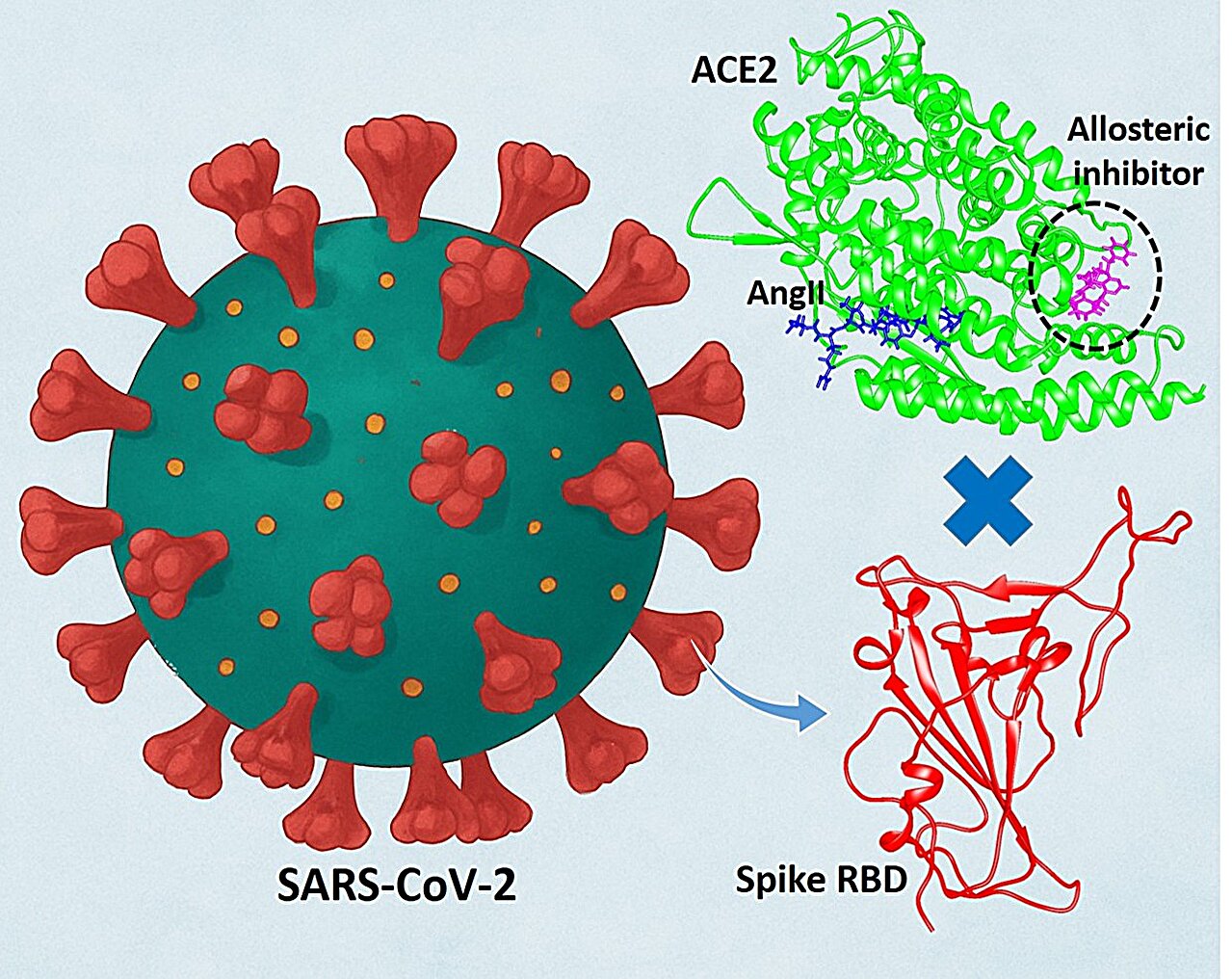
The illustration represents a Áce2-attached to the-up inhibitor, blocking the interaction between the SARS-COV-2 Spike and Ace2 protein. Simultaneously, the inhibitor improves the union of Ace2 to its natural substrate, angiotensin II, preserving its physiological function. Credit: Saroj Kumar Panda
At the beginning of the pandemic, most research, including ours, focused on the design of medications that could block the virus spike protein. This was a first logical step, but as we have seen, the virus is a mobile goal. It was quickly evolving, and the new variants acquired resistance due to changes in surface spike glycoprotein (protein S).
This highlighted a critical challenge: would our treatments continue to function as the virus continued to change? Instead of constantly pursuing new variants, we begin to ask, what would happen if we focus on how the human body responds to the virus, instead of just pointing to the virus itself?
This simple but powerful idea became the focus of our research, which we are proud to have recently published in it Physics of Physical Chemistry diary.
Change approach: go to the guest, not the virus
Instead of pursuing the virus directly, we decided to explore a new idea: go to the human protein that mediates the entrance of the virus into the cells of our body. This led us to the angiotensin-2 (ACE2) converture enzyme, the critical protein of the “door door” the kidnappings of the virus to begin its invasion. Ace2 is present on the surface of many human cells, especially in the lungs, and plays a crucial role in the regulation of blood pressure and heart health. Unfortunately, SARS-COV-2 kidnap this protein as its point of entry into cells.
This raises a significant challenge: blocking Ace2 completely is not a good option, since it is too important for normal body functions. So, our goal was: can we make it more difficult for the virus to use ACE2 without disturbing its vital role in our bodies?
Find a hidden switch
To answer this, we use a combination of powerful computational chemistry techniques. Our research adventure deviated from conventional approaches by not trying to block the ace2 viral junction domain. Instead, we use a computational alosteria approach to discover the “alestro” hidden protein site. This alkal site acts as a kind of molecular switch that, when triggered, can modulate the way the entire protein behaves.
Using molecular dynamics simulations (MD), we could visualize and study ACE2 and the interaction of the atomic virus. Our simulations showed that a small molecule modulator could bind to this newly discovered alostroi pocket, which is far from the primary virus interaction site. Free union energies calculated using the MM/PBSA method confirmed that our main compounds are favorably bind to this toostric pocket, which demonstrates the thermodynamic viability of our approach.

(A) The binding pose of SB27012 in the RBD complex 2 -Spike and a magnified representation of the designated to the designated toilets, (b) binding poses of three molecules SB27012 to 0 and 1000 NS derived from a non -affectionate MD simulation and (c) its RDF analysis and distance with respect to the designated waste. Credit: Physics of Physical Chemistry (2025). DOI: 10.1039/D5CP01740H
The double service advantage: hinder virus while helps the host
When an adequate small molecule joins this at the end of Ace2, it causes a conformational change in ACE2. This change mainly affects global alosteria, which is critical for the interaction of protein with viral spike glycoprotein. This conformational change weakens the union between ACE2 and the viral spike protein, which makes it more difficult for the virus to hook and infect a cell.
True innovation, however, is that this conformational change does not inhibit the normal function of ACE2; In fact, our simulations and calculations confirmed that it improves it. The toostical modulation increases the catalytic activity of Ace2 with its natural substrate, angiotensin II (Angii), a key part of blood pressure regulation. So, instead of blocking Ace2, we “push it” gently in a way that helps us and hinders virus.
This mechanism is analogous to adjusting a block: the original key (angiotensin II) is further better, but a copy (the virus) no longer works. Our work demonstrated that this strategy of alostric modulation could be an effective way to inhibit viral entry.
Looking to the future: a more resistant defense
The advantage of this approach aimed at host is significant. Most antiviral strategies aim to block the virus itself, but viruses like Sars-Cov-2 mutate rapidly; A drug that works today can fail tomorrow. Directing human protein viruses depends that it is much more difficult for viruses to escape treatment.
This makes our approach potentially more durable against future concern variants. By strategically understanding and strategically manipulating our own cellular machinery, we can build a more resistant defense against viral threats.
A team effort and personal trip
I am especially proud that this research has been carried out in Iiser Berhampur, a young and growing research institute in India. Our laboratory, the physical biomolecular research laboratory, uses advanced computational tools to explore therapeutic design against different pathogenic objectives.
This work highlights the power to think differently and underlines the value of fundamental science to address real world challenges. It was a real team effort that involved me, Pratyush Pani (Ph.D. Scholar), and our group leader, Dr. Malay Kumar Rana. Together, we combine our experience and curiosity to overcome the limits of what is possible through computational biology.
Science does not always need to fight hard; You just need to be intelligent.
This story is part of X science dialoguewhere researchers can inform the results of their published research articles. Visit this page To obtain information about the science X dialogue and how to participate.
More information:
Pratyush Pani et al, modulating the functionalosterio of the protein of the host cell receptor act2 to inhibit the viral entry of Sars-Cov-2, Physics of Physical Chemistry (2025). DOI: 10.1039/D5CP01740H
Dr. Saroj Kumar Panda is a postdoctoral research associate in the Department of Chemistry and Biochemistry of the University of Texas in Arlington, USA. His research focuses on the therapeutic design aimed at several pathogenic objectives and exploring enzymatic reaction mechanisms using advanced computer techniques. Dr. Panda obtained his Ph.D. Of the Department of Chemical Sciences, Indian Institute of Scientific Education and Research (Iiser) Berhampur under the tutoring of Dr. Malay Kumar Rana.
Citation: How the modulation of the human protein ACE2 could stop the entrance of Coronavirus (2025, August 15) recovered on August 24, 2025 from https://phys.org/news/2025-08-human-protein-ce2-modulation-ingy.html
This document is subject to copyright. In addition to any fair treatment with the purpose of study or private research, you cannot reproduce any part without written permission. The content is provided only for information purposes.
#modulation #ACE2 #human #protein #stop #entry #Coronavirus