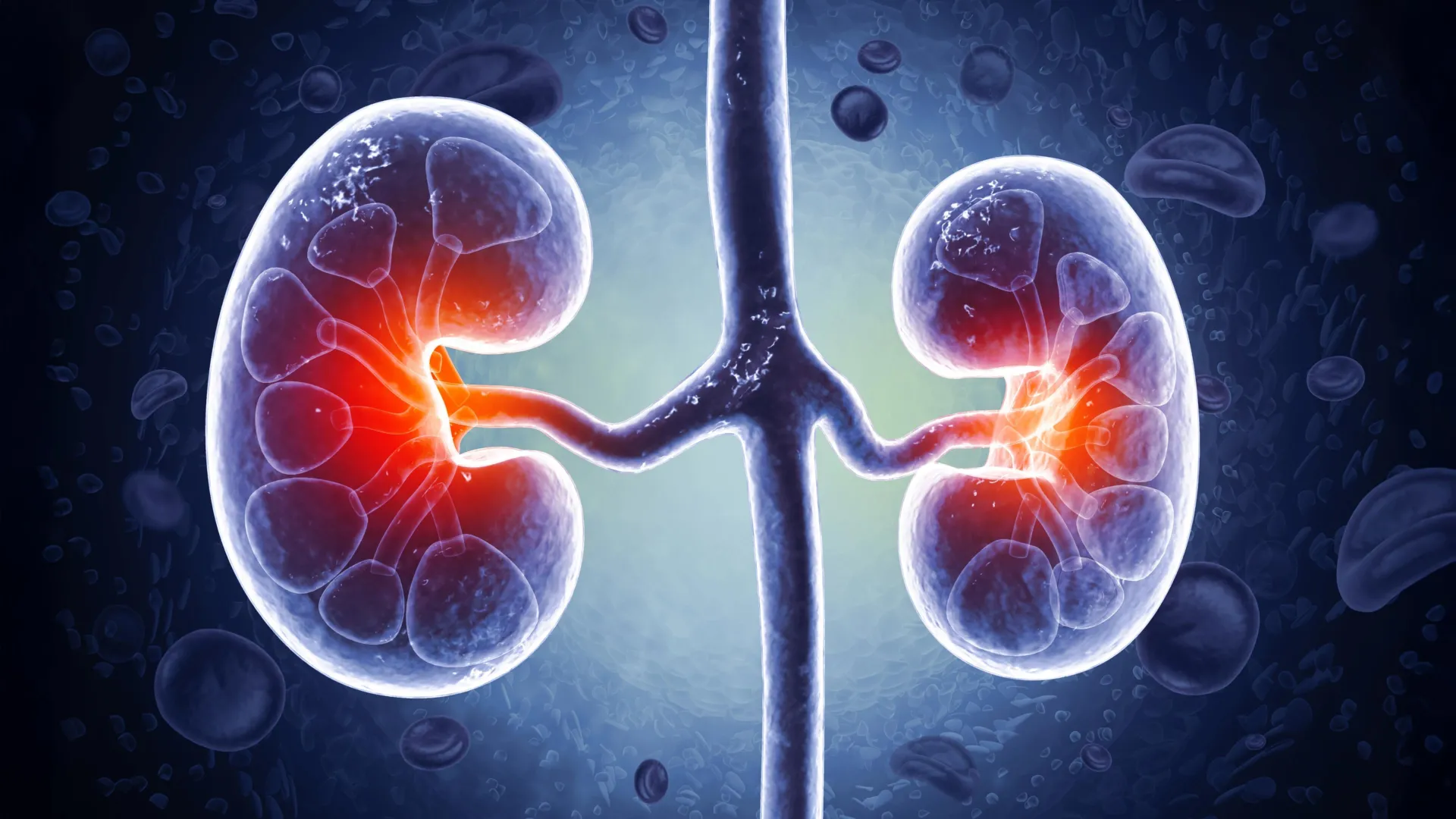
A molecule made by bacteria in the intestine can make a trip to the kidneys, where it establishes a reaction in inflammation chain, scars and fibrosis, a serious complication of diabetes and a main cause of renal failure, according to a new study of researchers from the University of Illinois UrbanoS Champaign at the University of Japan in Japan.
After finding high levels of Corisina, a small peptide produced by Staphylococcus Bacteria in the intestine: In the blood of patients with diabetic renal fibrosis, researchers used computer simulations and tissue and mouse experiments to trace how the corisine affects the kidneys, how it comes from the intestine and a possible method to counteract it with antibody treatment.
“Our previous studies showed that the Corisina can damage the cells and worsen the tissue scars and fibrosis in other organs, so we suspect that it could be a hidden driver of renal fibrosis,” said Illinois animal science professor, Isaac Cann, who directed the study with the immunology professor at the University of Mie, Dr. Esteban Gabazza. Cann and Gabazza are affiliated by the Carl R. Woese Institute of Genomic Biology in Illinois. “Our new findings suggest that Corisin is a guilty hidden behind the progressive renal damage in diabetes, and that blockage could offer a new way of protecting renal health in patients.”
The researchers published their findings in the magazine Nature communications.
Diabetic renal fibrosis is an important cause of renal failure worldwide, however, key drivers have remained a mystery, and no treatment can stop the process, said Dr. Taro Yasuma from the University of Mie, a medical doctor and the first author of the manuscript.
“Many people with long -standing diabetes eventually develop renal fibrosis, and once it progresses, there are limited options beyond dialysis or kidney transplant.
The researchers began detecting the blood and urine of patients with diabetic renal disease. They discovered that patients had significantly more corisine than their healthy counterparts, and that the amount of corisine in the blood correlated with the reach of renal damage.
Seeing the same results in mice with renal fibrosis, the researchers tracked what Corisin was doing in the kidneys of the mice. They discovered that the Corisina accelerates aging in renal cells, triggering a chain reaction of inflammation to cell death to a scar tissue accumulation, which eventually results in the loss of renal function and the worsening of fibrosis.
But how was Cornisin from the intestine to the kidneys? Cann and Gabazza groups collaborated with the U. of I. The group of the professor of chemical and biomolecular engineering Diwakar Shukla to produce computer simulations and laboratory experiments to follow Corisin’s trip from the intestine to the bloodstream. They discovered that Corisin can join albumin, one of the most common proteins in the blood, and mount it through the bloodstream. When it reaches the kidneys, Corisin separates from albumin to attack the delicate structures that filter blood and urine.
To confirm that Corisin was the main culprit of renal damage, the researchers gave the antibodies of the mice against Corisin. They saw a dramatic reduction in the speed of renal damage.
“When we treat mice with an antibody that neutralizes the Corisina, it slowed the aging of renal cells and greatly reduced renal scars,” said Gabazza, who is also an attached professor of animal sciences in Illinois. “While this antibody is not currently approved for use in humans, our findings suggest that it could become a new treatment.”
Next, the researchers plan to test treatments against anticorisin in more advanced animal models, such as pigs, to explore how they could adapt for safe use in humans. The U. of I. and the University of Mie have a disclosure of joint invention in Corisin antibodies.
“Our work suggests that blocking the Corisina, either with antibodies or other directed therapies, could slow or prevent renal scars in diabetes and, therefore, improve the quality of life of patients,” said Cann.
This study was supported by the Japan Science and Technology Agency, the Japan Society for the Promotion of Science, the Takeda Science Foundation, the
The Japan Diabetes Association for Education and Diabetes Care, the Innovation Research Scholarship of Japan Eli Lilly, the Daiwa Security Foundation and the Charles and Margaret Levin Family Foundation. Cann is also a professor of Microbiology and Nutritional Sciences and a member of the Eastern and Pacific Asia Studies Center in Illinois.
#Hidden #intestinal #molecule #kidneys