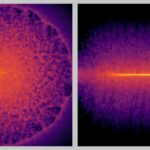
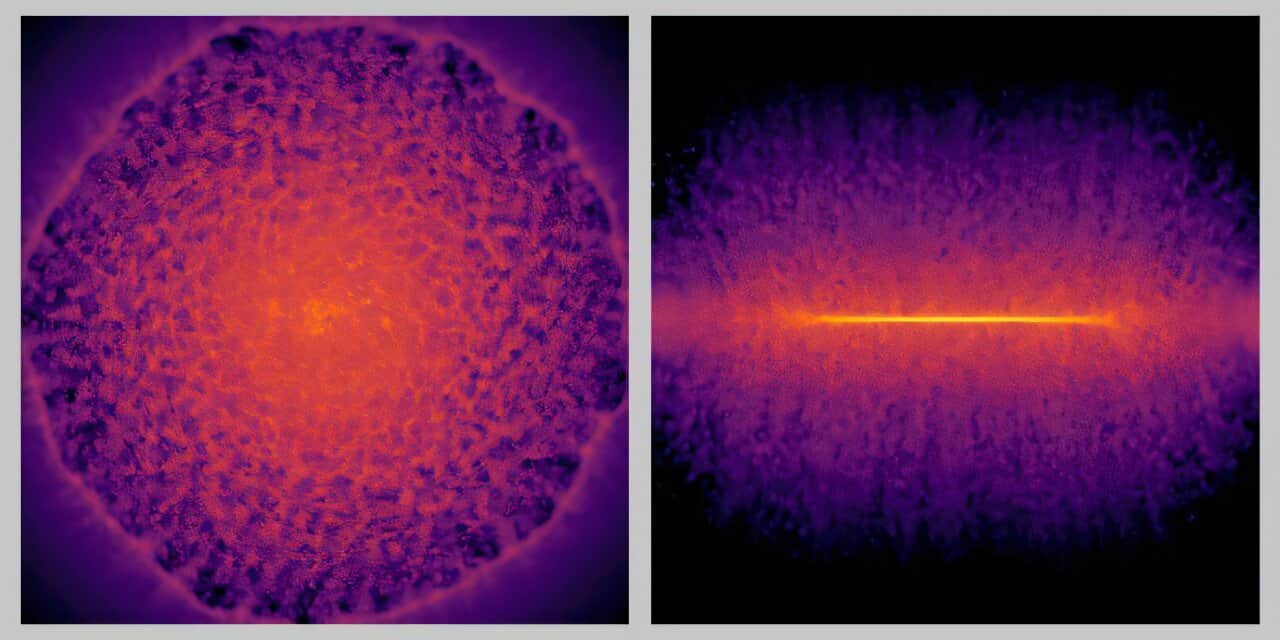
When scientists squeezed a drop of water between two diamonds to withstand pressures tens of thousands of times greater than Earth’s atmosphere, something unexpected happened. The liquid solidified at room temperature, not into any known form of ice, but into a new crystalline phase.
This never-before-seen structure, called ice XXI, was discovered by researchers led by Geun Woo Lee at the Korea Research Institute of Standards and Sciences (KRISS) in collaboration with scientists from Europe’s XFEL and DESY in Germany.
It is the twenty-first form of ice ever identified. Scientists have already cataloged an astonishing variety of structures: hexagonal, cubic, amorphous and even superionic. Each shape emerges under unique combinations of temperature and pressure. Ice XXI now joins that expanding family, demonstrating that the solid state of water is much more diverse than anything found in a freezer.
“Water is one of the most mysterious materials in the universe,” Lee said. “Why do two such simple elements form so many different types of phases? We think there are still different ones, [unknown] ice crystal phases: When we say unknown, we mean it has not been discovered yet, but it may exist.”
Crush water until it turns into glass

To create ice XXI, the researchers did not chill the water, but crushed it. They used a dynamic diamond anvil cell, a device that acts like a microscopic hydraulic press capable of generating two gigapascals of pressure, about 20,000 times atmospheric pressure.
In each experiment, the scientists squeezed water for just 10 milliseconds and then released it over the course of one second. They then repeated the process more than a thousand times. As the cell pulsed, X-ray bursts from the European XFEL recorded the smallest changes in the structure, frame by frame, every microsecond.
“The rapid compression of water allows it to remain liquid up to higher pressures, where it should have already crystallized into ice VI,” Lee explained.
Normally, at these pressures, water solidifies, forming ice VI, a dense phase already believed to exist within the icy moons Ganymede and Titan. But the rapid compression created a detour – what Lee calls a “hidden path” – through which the liquid briefly gathered into an entirely different crystal. That’s where ice XXI appeared, which lived only tens of microseconds before transforming into ice VI.
“Using the unique X-ray pulses from the European
The strange geometry of ice XXI

When examined under the microscope, ice XXI is made up of tetragonal crystals. It is essentially a geometric structure that repeats itself in blocks of 152 water molecules, making it unlike any other known ice.
Using PETRA III and its high-energy X-ray photons, the team determined the atomic arrangement. Ice XXI consists of very compact, highly ordered and surprisingly large unit cells. “The structure in which liquid H₂O crystallizes depends on the degree of supercompression of the liquid,” Lee explained.
In the paper, the researchers describe the formation of Ice XXI as “a metastable phase that forms along an alternative crystallization pathway within the pressure field of Ice VI.” In other words, it exists in a precarious balance between being liquid and turning into another type of ice.
This phenomenon is a key clue to how water behaves in extreme conditions. “Our findings suggest that a larger number of high-temperature metastable ice phases and their associated transition pathways may exist,” said DESY physicist Rachel Husband. “It potentially offers new insights into the composition of icy moons.”
Inside those moons, the water is not frozen like in the freezer. It is crushed under megabars of pressure, where molecules clump together in exotic arrangements like ice VI, VII and even XVIII. So each layer conducts heat and electricity differently. Ice XXI could represent an intermediate step, forming and melting as the outer layers of these moons shift and crack.
Why this matters
Ice XXI may exist only for microseconds in the laboratory, but its implications extend throughout the solar system. On distant moons, where pressures and temperatures swing wildly, these fleeting crystalline forms could shape how ice sheets stack up, how oceans circulate under miles of thick ice, and even how tectonic-like forces propagate through their crusts.
It also redefines the way scientists think about phase transitions in water, a substance so common that we barely realize how strange it really is. Every glass of water is a shapeshifter waiting to happen.
“There are many questions [as to] “How such a simple material produces many different crystalline phases,” Lee said. “Researchers want to understand the detailed pathways of crystallization from water to ice.”
Sakura Pascarelli, Scientific Director of the European XFEL, summed it up: “It is fantastic to see another great result from our Water Call, an initiative that invites scientists to propose innovative studies on water. We look forward to many more interesting discoveries in the future.”
The findings were reported in the journal. Nature materials.
#Scientists #discover #water #turns #type #ice #exist #alien #worlds