The development of safe and effective therapies for a disease often has been. However, Covid-19 pandemic needs immediate therapy since hundreds of millions of people have been infected and millions have died. The reuse of existing medications that have already been approved for another disease have the potential to provide COVID-19 therapeutics more quickly.
This approach was adopted for COVID-19 by an inter-institutional team of researchers: Professor Gaetano Montelion RESSELEAR POLYTECHNIC INSTITUTE (RPI); Professor Kris White, Professor Adolfo Garcia-Sastre, Dr. Romel Rosales, Dr. Lisa Miorin, Elena Moreno and Thomas Kehrer at the Icahn School of Medicine in Mount Sinai; and Professor Robert Krug at the University of Texas in Austin.
These researchers tested ten hepatitis C drugs available (HCV) for their ability to suppress the replication of SARS-COV-2, the virus caused COVID-19, and showed that several of these HCV medications have potential as therapeutics for COVID -19. In particular, four of these VHC medications improve the antiviral activity of SemDesivir, the only antiviral drug approved for COVID-19, up to 10 times. This study was published in the magazine Cellular reports.
Profes SARS-COV-2 caused by Covid-19 “disease.
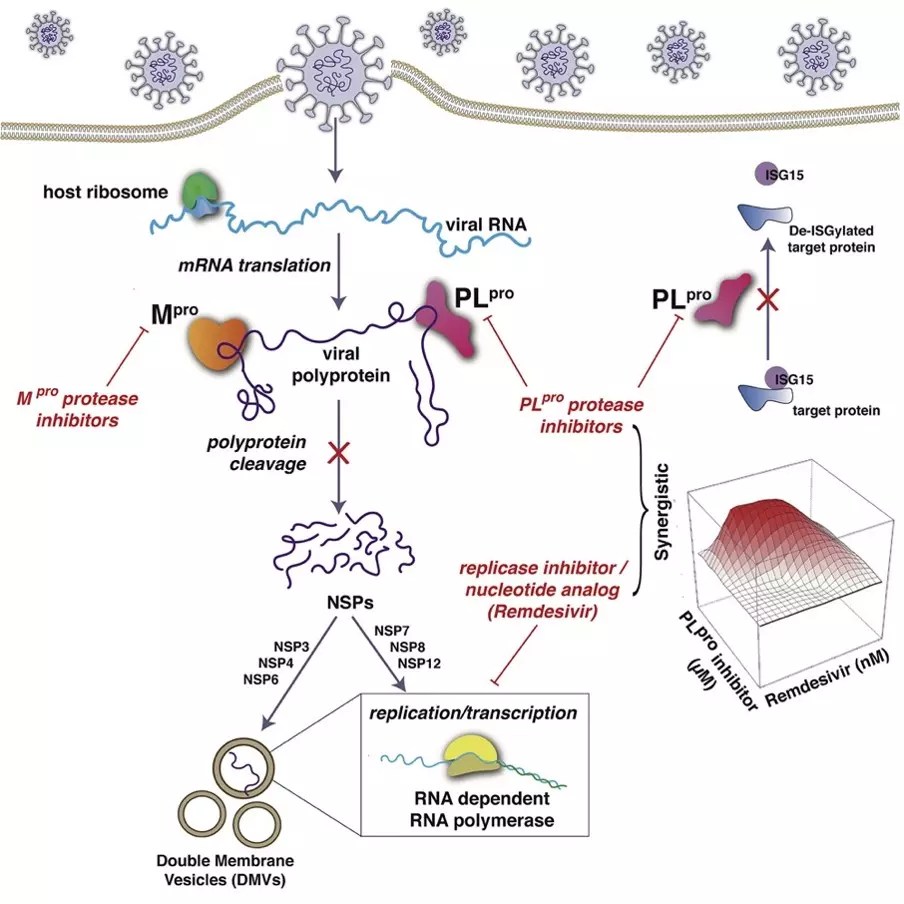
The motivation for this study was the observation of RPI researchers that there is a surprising similarity between HCV protease structures and one of Sars-COV-2 proteases, called the main protease. These viral proteases play an essential role in viral infection. They wondered if existing medications that bind and inhibit a VHC protease could also join and inhibit this SARS-COV-2 protease.
Using a supercomputer to model how medications bind to viral proteins, RPI researchers predict that ten HCV medications could comfortably join this SARS-COV-2 protease. In addition, they showed that seven of these medications inhibited this SARS-COV-2 protease
The Mount Sinai research team then tested these seven medications in a safe biocontent center for its ability to inhibit the replication of the Sars-COV-2 virus in mono and human cells cultivated in culture. The seven VHC drugs inhibited virus replication.
In subsequent experiments, the researchers were surprised to discover that four of the VHC medications (Simeprevir, Vaniprevir, PARITREPREVIR AND GRAZOPREVIR) inhibited the other SARS-COV-2 protease called PlProtease. These results proved to be very important.
Sedesivir, the only antiviral drug approved for COVID-19, is directed to a protein of the coronavirus Sars-Cov-2 called RNA polymerase that synthesizes viral RNA. Because the two SARS-COV-2 proteases are necessary for the production of a functional POLIMERASA RNA, HCV drugs can be expected to improve the effectiveness of selecting in the inhibition of virus replication. The researchers showed that only the four VHC medications that are directed to the unexpected SARS-COV-2 Plprotease increase the effectiveness of selecting, up to 10 times. In contrast, VHC medications that are directed only to the main protease SARS-COV-2 did not improve the effectiveness of selecting.
As indicated in the document: “VHC drugs that are strongly synergistic with sedivir are more relevant to the objective of the present study. Reuse medications may not have enough inhibitory activity on their own to achieve clinical efficacy. The synergy with sedesivir increases the power of both the proposed reuse medications and the sedesivir. “
“PLPRO identification as an antiviral objective that has a synergistic effect with Sedesivir is a very important finding. We hope that this work encourages the development of specific SARS-COV-2 PLPRO inhibitors for inclusion in therapies combined with polymerase inhibitors to produce a highly effective antiviral cocktail that will also avoid the increase in resistance mutations, “said Kris White .
As Adolfo Garcia-Sastrre emphasized, “the combined use of semDesivir with a inhibitor of
The PlProtease for the treatment of COVID-19 would also reduce the possibility of selecting virus resistant to SARS-COV2 “.
HCV medications are administered orally, while semi -man -administered intravenously. Consequently, the treatment of patients with COVID-19 with a combination of semdesivir with a HCC medication would have to take place in hospitals. The results of this document firmly support that the creation of a clinical trial to evaluate this combination of medications in hospitalized patients.
In addition, as Krug said: “Our goal is to develop a combination of oral medications that can be administered to outpatients before they are sick enough to require hospitalization. For this purpose, it is necessary to identify oral drugs that inhibit SARS-COV-2 polymerase to develop an effective outpatient treatment. “
Main image magazine and credit reference:
BAFNA, K., WHITE, K., HARISH, B., ROSALES, R., RAMELOT, TA, ACTON, TB, MORENO, E., KEHRER, T., MIORIN, L., ROYER, CA, GARCÍA, GARCÍA-SASTR , A., Krug, RM and Montelione, GT (2021). Hepatitis C virus drugs that inhibit Sars-Cov-2 papain protease synergy with sedivir to suppress viral replication in cell culture. Cell Reports, 35 (7), 109133. https://doi.org/10.1016/j.cerep.2021.109133

#Hepatitis #medications #increase #antiviral #activity #selecting #COVID19