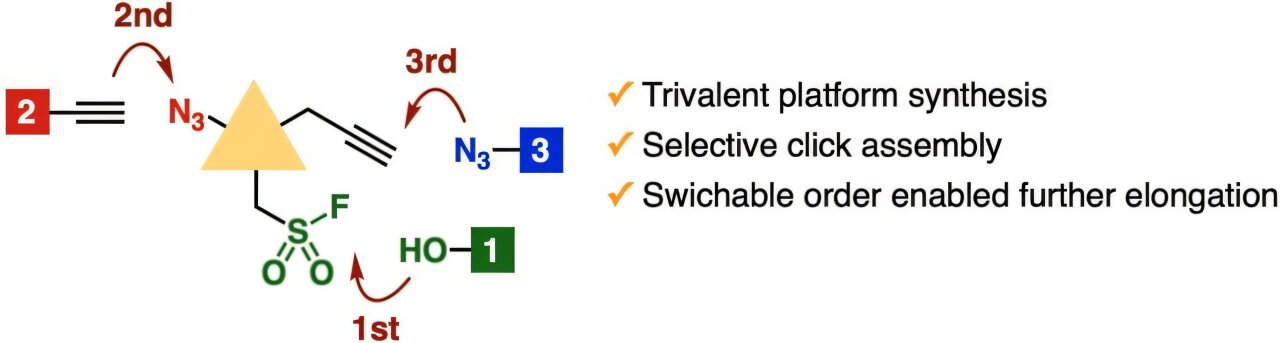
The proposed platform for triple click chemistry has three functional groups that can be attacked independently for replacement when choosing an appropriate reaction partner. This platform is compatible with sequential and versatile reactions of a single container that produce multi-triazols. Credit: Dr. Suguru Yoshida/University of Sciences of Tokyo, Japan Source Link: Pubsc.org/en/content/articleanding/2025/cc/d4CC06585A
The average molecules with a molecular weight of more than 1,000 are difficult to synthesize due to multiple steps and a nature that requires a lot of time, demanding the development of a new approach that can overcome these disadvantages.
Click Chemistry has become an essential tool in applied chemistry due to its simplicity, efficiency and versatility. This approach to chemical synthesis allows the rapid and reliable junction of small molecules in larger and more complex structures, often with minimal lateral reactions and by -products.
By definition, click chemical reactions are highly selective and efficient, which makes them ideal for creating specific compounds in a controlled and predictable way.
When taking this idea two steps further, chemicals have been developing molecular platforms that allow triple click chemistry, the development of stable molecules with three different functional groups that serve as different and objective reaction sites.
Although these “trivalent” platforms allow the efficient synthesis of complex compounds, the selective formation of triazoles using platforms with azid and alquino remains remains an unsolved challenge.
In this context, a research team led by the Associate Professor Suguru Yoshida of the University of Sciences of Tokyo (TUS), Japan, set out to develop new trivalent platforms capable of producing highly functional triazols.
The team assured the coordination with the United Nations Sustainable Development Goals (SDG) -SDG 3 (good health and well-being), SDG 7 (affordable and clean energy) and SDG 9 (industry, innovation and infrastructure).
The study, which was published in Chemical communications On January 7, 2025 he was co -author of Mr. Takahiro Yasuda, a master’s student, and Mr. Gaku Orimoto, who completed a master’s degree in 2023, both of you.
The researchers managed to create stable trivalent platforms for triple click chemistry, thanks to a longer linker in the central scaffolding. The research team showed how a wide variety of molecules could be produced by sequentially pointing to each functional rest on the trivalent platform.
For example, they took advantage of the sulfur exchange reaction -fluoruro to aim at the rest fluorosulfonyl and produce different alcohols with high yields without affecting the remains of Azida and Alquino.
Then, they performed various transformations in the rest Azide, including distinctive stamps such as the cicy of Azida-Alcino catalyzed by copper, the azid-alquiline cycle promoted by the voltage and ligation of Bertozzi-Staudinger.
Finally, through a wide range of possible third transformations aimed at the remaining Alkyne, the researchers successfully synthesized complex triazols.
In particular, it was not strictly necessary to follow the order described above when it was addressed to each rest, since the researchers demonstrated triazol formations selectively in subsequent experiments. In addition to this, complex triazols could be obtained in a simple reaction of a single container.
“” The selective click reactions with molecules that have the remains of Azid target group under the target group. Adequate conditions, “explains Yoshida.
Triple click chemistry platforms developed in this study have important implications in several applied fields. For example, functionalized multi-triazols, which can be easily prepared with high performance, are valuable in drug development, material science and bioengineering. They are compatible with many biological objectives, such as enzymes and receptors, indicating possible pharmaceutical applications.
Bioactive average molecules synthesized through triple click chemistry can help recover from intractable diseases. In addition, they are important in the development of catalysis and materials, which serve as the basis for the design of polymers, sensors, coatings and coordination frames.
“Our ultimate goal is to create new molecules that will revolutionize life sciences, and we conceive this research as a method to assemble simple component molecules at once,” concludes Yoshida.
“The proposed method allows the simple synthesis of multifunctional molecules and a wide variety of medium -sized molecules, and we hope it will be widely useful in pharmaceutical science, medicinal chemistry, chemical biology and chemistry of materials.”
The proposed approach uses simple initial materials instead of complex materials, promoting sustainable pharmaceutical synthesis. In addition, the aspect that saves time of this approach can accelerate the research process.
In general, the efficient trivalent platform molecules presented in this study will help accelerate progress towards a more sustainable chemistry, hopefully lead to green synthesis protocols, better medical treatments and environmental and agricultural advances.
More information:
Takahiro Yasuda et al, three -step click assembly using trivalent platforms with sugar groups, ethinyl and fluorosulfonil, Chemical communications (2025). DOI: 10.1039/D4CC06585A
Citation: Click the Chemistry Method advances the development of drugs with an improved triazol synthesis (2025, February 11) recovered on February 18, 2025 from https://phys.org/news/2025-02-Click- chemistry-method-advances-drug.html
This document is subject to copyright. In addition to any fair treatment with the purpose of study or private research, you cannot reproduce any part without written permission. The content is provided only for information purposes.
#Click #chemistry #method #advances #development #drugs #improved #triazol #synthesis