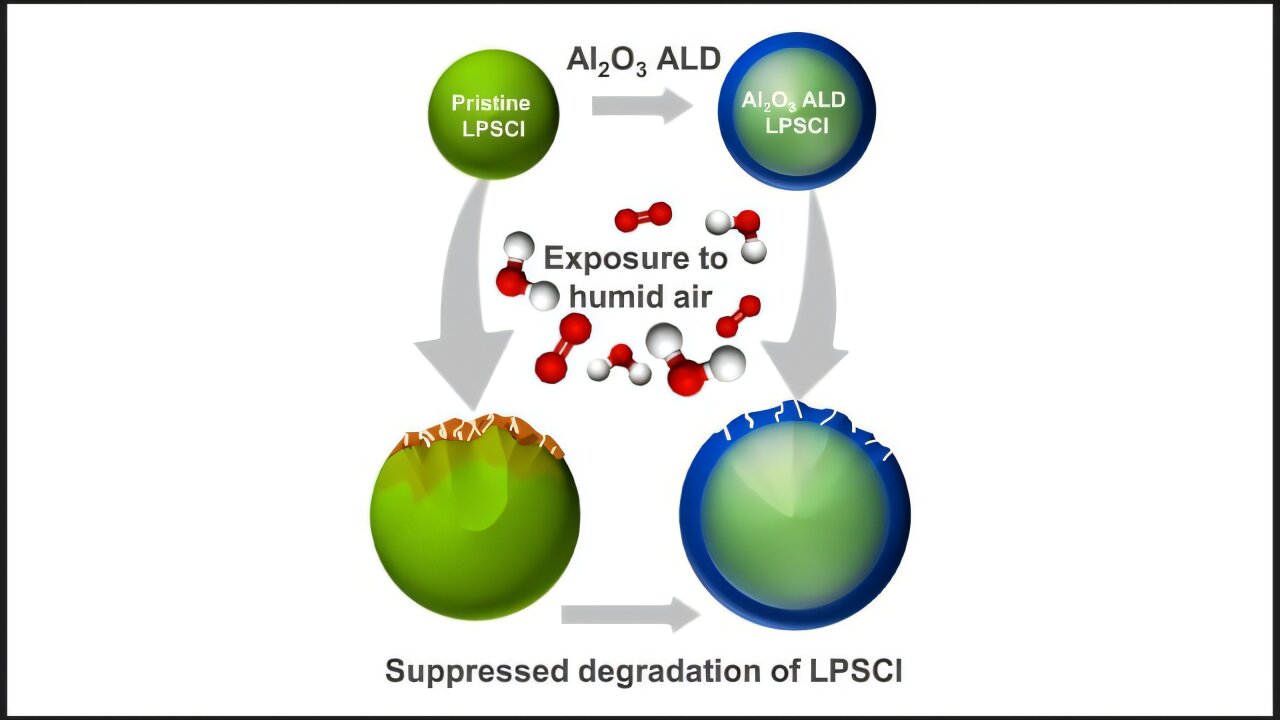
A comparison of LPSCI without coating (an electrolyte based on sulfide) with LPSCI coated with aluminum oxide when exposed to humid air, illustrating how the coating suppresses degradation. Credit: Taewoo Kim/Argonne National Laboratory
A thin layer in the form of glass could be the key to profitable solid state batteries.
In everyday life, we use many protective barriers: the sunscreen protects us from the sun, the umbrellas keep us dry in the rains and the baking gloves protect our hands from hot pans. Similarly, batteries need protection to prevent their internal components from breaking due to environmental exposure.
Within a battery, the electrolyte is the chemical that allows the electric charge to flow between its components. Solid state batteries (SSB) use solid electrolytes instead of liquids found in regular lithium ion batteries. Through the use of solid electrolytes, the SSB could revolutionize the energy storage industry by offering a better energy, safety and useful life density than those of lithium ions.
However, a great challenge for SSB is that solid electrolytes can decompose when exposed to atmospheric conditions such as moisture and oxygen. This challenge is particularly severe for solid electrolytes based on high performance sulfide, such as phosphorus sulfur lithium chloride (LPSCL). Making SSB with these materials requires maintaining a dry room below -40 ° C, which makes production expensive.
To improve chemical stability and make manufacturing more affordable, researchers of the National Argonne Laboratory of the United States Department of Energy (DOE) have developed a method to cover solid electrolytes based on sulphide. They use a process called atomic layer deposition (ALD) to apply a protective layer.
This coating improves the chemical stability of the electrolyte not only acting as a physical shield, but also modifying the electronic structure of the surface, resulting in materials that are more stable for moisture and oxygen. Results of this investigation They were published in ACS material letters.
“Our research shows that even a very thin coating, only a few nanometers thick, or approximately 100,000 times thinner than a human hair, can act as a strong barrier, maintaining the electrolyte intact and increasing its performance,” said Argonne Connell materials. “This advance can not only extend battery life, but it can also reduce manufacturing costs by allowing production in less controlled environments.”
The ALD process, commonly used to make computer chips, deposits a layer of aluminum oxide on electrolyte particles. Aluminum oxide is similar to glass, with many of the same properties.
“We have covered the solid electrolyte dust with an ultrafine layer similar to that of glass that prevents it from reacting with the atmosphere,” said Jeffrey Elam, a senior chemist and distinguished companion from Argonne. “This material can be so thin that it is less than an atomic layer, which means that it is thinner than the diameter of a single atom. At first, this result baffled us, but computational modeling helped discover an explanation.”
Peter Zapol, a computational scientist, explained: “Initially we thought that the coating was just a physical barrier, but we discovered much more about the electronic properties of the electrolyte. The ALD coating alters the electronic structure of the electronic structure of the electrolyte surface, which helps to suppress the degradation and maintain the conductivity of lithium ions.
The protective layer not only maintains the stable electrolyte, but also guarantees an efficient movement of lithium ions, which is essential for the battery operation.
In tests with high humidity and oxygen, comparable to environmental air, coated electrolytes worked much better than non -coated. Coated materials remained stable with little degradation, while non -coated showed a significant decomposition and atmospheric reactivity.
The ability to work with these materials in less controlled environments is a key advantage of this coating. The scientist of Materials Zachary Hood said that handling these materials in tougher conditions would simplify the manufacturing process.
“It would allow manufacturers to use the existing infrastructure, similar to what is used for lithium -ion batteries,” he said. “This would result in significant savings in the initial cost of the factories necessary to make batteries with these materials, while improving reliability, since there is less concern for the degradation of materials during assembly.”
The team is also working to expand this method. They are currently collaborating with a commercial partner to produce large amounts of the coated electrolyte for demonstration in larger format batteries.
While the equipment has achieved success with the current aluminum oxide coating, it recognizes that it is only one of the many possible coating chemicals. There are many others to explore, and future research will focus on these alternatives.
Other taxpayers to this work include Taewoo Kim, Aditya Sundar, Anil Mane, Francisco Laguna, Jordi Cabana and Sanja Tepavcevic of Argonne; Khagesh Kumar, who is affiliated with Argonne and the University of Illinois in Chicago; and Nelam Sunariwal of the University of Illinois in Chicago.
More information:
Taewoo Kim et al, suppressing the atmospheric degradation of solid electrolytes based on sulfide through ultratine metal oxide layers, ACS material letters (2024). DOI: 10.1021/ACSMATERIALSLETT.4C01923
Citation: Solid state batteries obtain an impulse with a new protective coating (2025, September 10) recovered on September 18, 2025 from https://phys.org/news/2025-09-solid-state-batteries- Boost-coating.html
This document is subject to copyright. In addition to any fair treatment with the purpose of study or private research, you cannot reproduce any part without written permission. The content is provided only for information purposes.
#Solid #state #batteries #obtain #boost #protective #coating