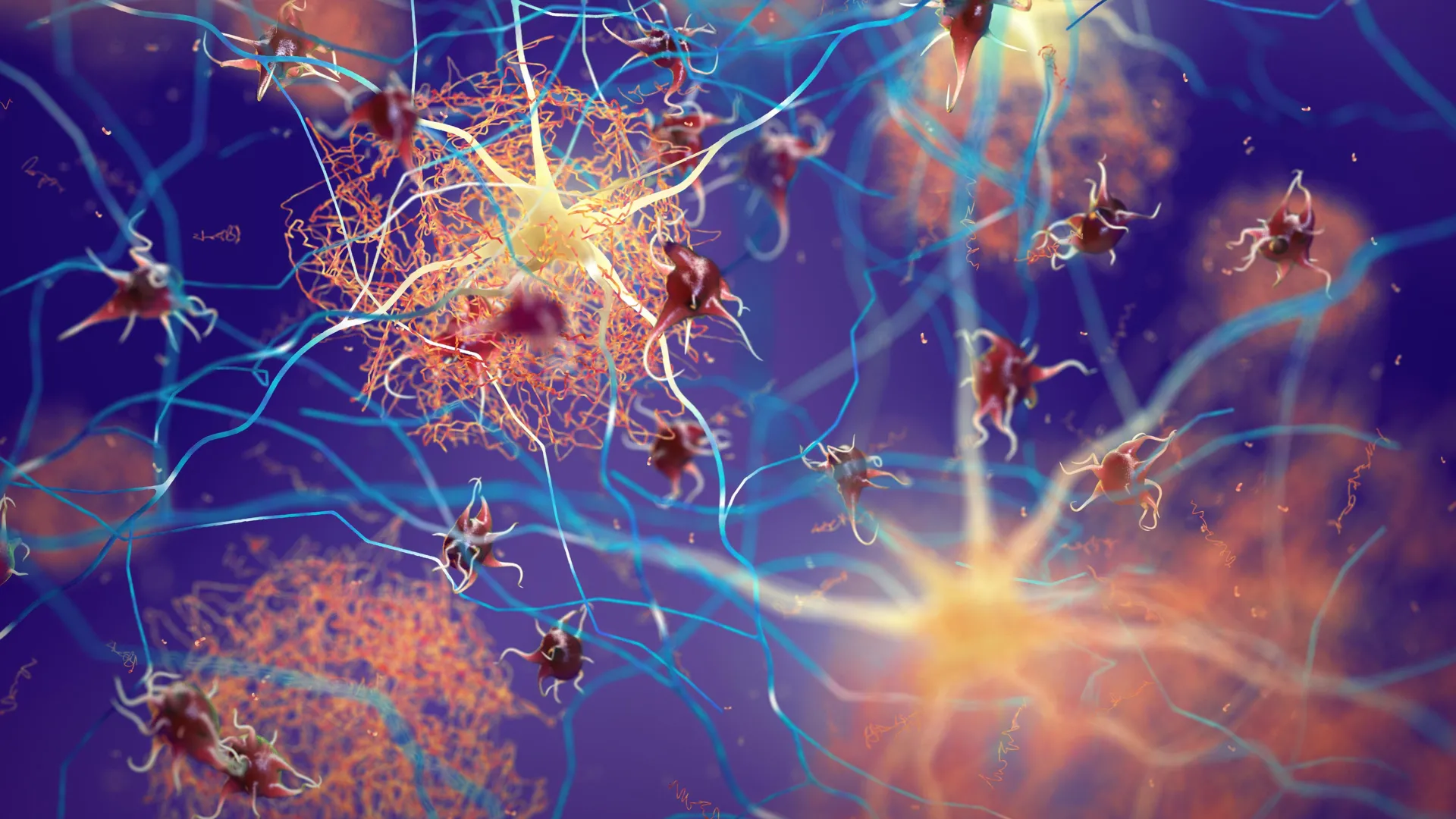
Researchers at Tokyo Metropolitan University have turned to concepts from polymer physics to better understand a central feature of Alzheimer’s disease: the formation of tau protein fibrils. Their work revealed that these fibrils do not appear suddenly. Instead, they emerge after large clumps of tau proteins begin to assemble in solution, a process that resembles the crystallization of polymers. When the scientists disrupted these early clusters, the fibrils did not form, suggesting a promising direction for new strategies against neurodegenerative diseases.
Alzheimer’s disease (AD) remains one of the most difficult medical challenges, especially as global populations continue to age. Scientists have long focused on pharmacology and traditional biomedical approaches. However, the complexity of AD has led researchers to explore knowledge from other scientific fields that can open new avenues for understanding the disease and designing treatments.
How polymer behavior helped explain Tau protein fibrils
Led by Professor Rei Kurita, the research team applied ideas from how polymers organize into crystals. Polymers, which are long chains of repeating molecular units, often do not crystallize by simply adding one chain at a time. Instead, they pass through intermediate precursor structures before forming an ordered crystal. Building on this concept, scientists examined tau proteins in solution and discovered that fibril formation (fibrillization) is also preceded by a precursor stage. In this case, the precursor is a loose assembly of tau proteins measuring just tens of nanometers. Techniques including small-angle X-ray scattering and fluorescence-based analyzes confirmed the presence of these structures.
A key discovery was that these precursors are not rigid but are soft, temporary clusters. The researchers were able to dissolve them by altering sodium chloride levels in the presence of heparin, a natural anticoagulant. When the clumps were broken up or prevented from forming, the solution produced almost no tau fibrils. The team suggested that this effect occurs because increasing concentrations of charged ions reduces the strength with which tau proteins interact with heparin. According to their explanation, this change improves electrostatic “sensing”, making it more difficult for molecules to find each other and form groups.
A new therapeutic direction for Alzheimer’s and beyond
These findings point to a potential change in the way scientists approach treatments for AD. Instead of trying to break up the final fibrils, therapies could aim to stop the reversible precursor stage before harmful structures develop. This approach could influence not only AD research but also efforts to understand other neurodegenerative diseases, including Parkinson’s disease.
This work was supported by JST SPRING program grant number JPMJSP2156, JSPS KAKENHI grant numbers 22K07362, 25K21773, 24H00624, 22H05036, 23K21357, 25K02405, 23H00394, 23KK0133, and 20H01874, JST Moonshot R&D program grant number JPMJMS2024 and AMED grant number 24wm0625303 and 25dk0207073.
Tau protein fibrils
Tau protein fibrils are abnormal bundles of tau proteins that assemble inside neurons when tau loses its normal shape and function. Under healthy conditions, tau functions as a stabilizing support beam, helping to maintain microtubules that allow nutrients and signals to move through nerve cells. When tau misfolds, it begins to clump together into long, fibrous aggregates known as fibrils. These structures interfere with the cell’s internal transport system and are strongly linked to the cognitive impairment seen in Alzheimer’s disease and other neurodegenerative conditions. Because fibrils grow from smaller, early-stage tau pools, researchers are increasingly focusing on blocking these initial steps to prevent further damage.
#Scientists #melt #early #protein #clumps #stop #Alzheimers #damage