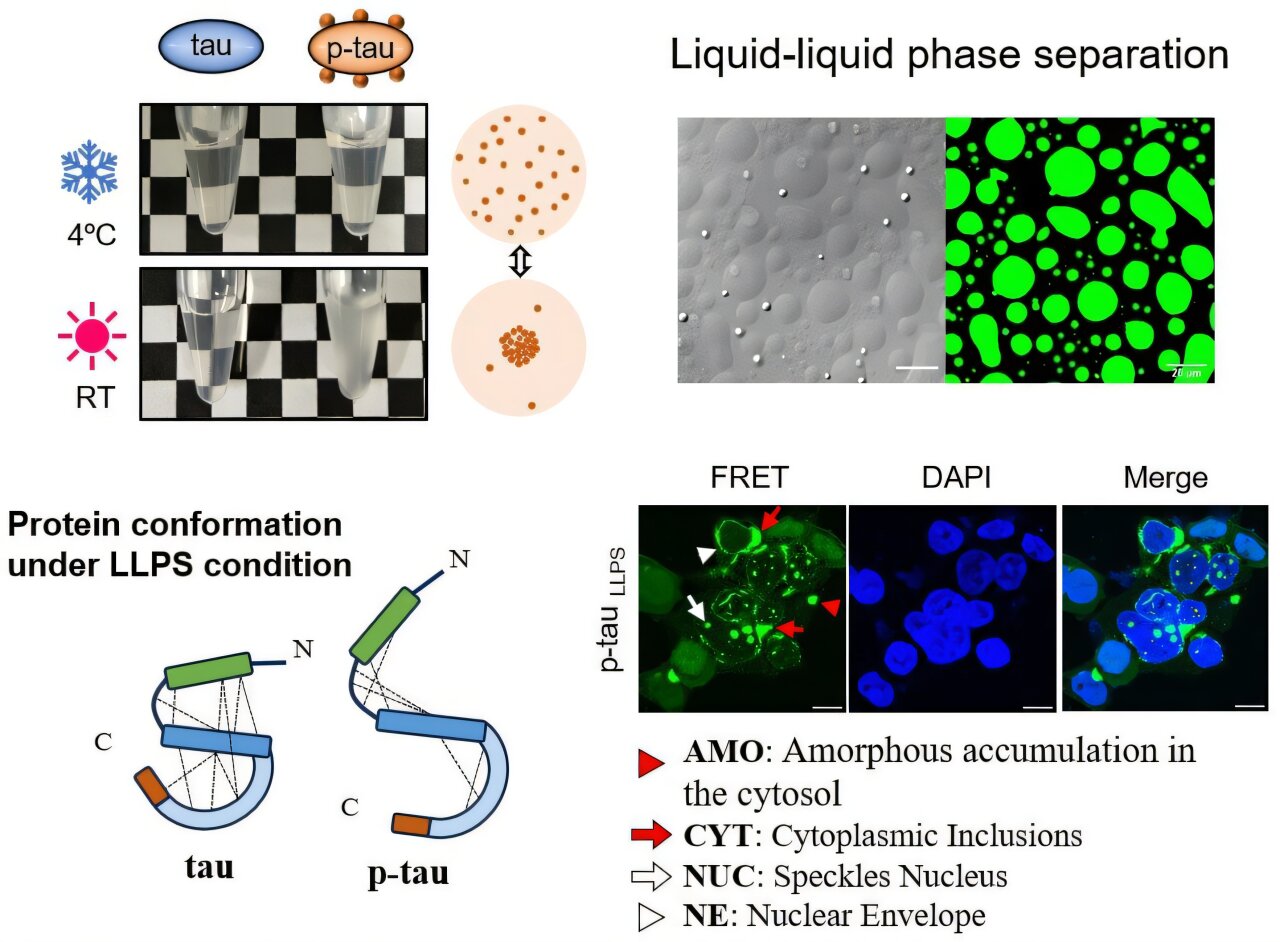
Tau phosphorylation makes its structure more flexible and allows you to form dynamic drops of liquid when the temperature increases. Tau in this state can trigger the intracellular tau to add near the nuclear envelope. Credit: National University of Taiwan
In a study published in it Journal of the American Chemical SocietyScientists explore how a cerebral protein called Tau changes their behavior when a small chemical called phosphate joins it, a process known as phosphorylation.
Under normal conditions, Tau helps maintain healthy brain cells, but when it begins to group, it is linked to neurodegenerative diseases such as Alzheimer’s disease.
The research team compared two Tau forms: one with united phosphate groups (called Fosphorylated or P-tau) and another without. Exceptionally, they designed bacteria to produce P-tau using a specialized design called Pimax.
They discovered that only P-tau, not regular, could form spontaneously small drops, size micrometers, through a process known as separation of liquid-liquid phases (LLP), which is similar to how the oil is separated from the water.
This drop formation naturally occurred as the temperature changed cold (4 ° C) at room temperature and was driven by electrostatic and hydrophobic interactions. Surprisingly, the drops disappeared when cooling, demonstrating that the process is reversible.
Other experiments revealed that joining only a few groups of phosphate, between two and nine, was enough to make Tau more flexible and folded freely, allowing him to form drops.
The team also discovered that RNA can induce the formation of drops both in Tau and P-tau, but unlike the reversible self-taught of P-tau, the RNA-driven process is irreversible and occurs in a different timeline.
Interestingly, LLPS’s training capacity does not lead to the formation of amyloid fibrils. Neither tau nor p-tau form amyloid fibrils on their own; Trustful agents such as dextran sulfate or RNA were required.
Surprisingly, the Fibrils Tau/P-Tau induced by Dextran, the Fibrils Tau/P-Tau induced by RNA and the Tau/P-Tau conditions in conditions of LLP led to the aggregation of TAU fragments in cellular locations other than the biosensors cells.
In particular, only P-tau in conditions of LLP induced aggregation around the cell nucleus, a pattern that looks a lot like what is observed in the brains of Alzheimer’s patients. This new observation can provide important clues about how the disease originates.
“This is a significant discovery,” said Professor Rita P. – Y. Chen. “Our results demonstrate how a simple chemical modification, phosphorylation can fundamentally alter Tau’s behavior in cells, which offers new ideas about the first events of Alzheimer’s disease.”
“Currently, there is no reliable mouse model that completely imitates the human condition. We are now working on the development of a new mouse model that reflects more closely the pathological characteristics observed in Alzheimer’s patients,” he said.
More information:
Mohammadreza AllahyartTorkaman et al, self -preservation induced by phosphorylation versus complex coacervation assisted by TAU protein RNA, Journal of the American Chemical Society (2025). DOI: 10.1021/JACS.4C14728
Citation: Phosphorylation allows Tau protein to form reversible drops, offering information about early Alzheimer (2025, April 17) recovered on April 26, 2025 from https://phys.org/news/2025-04-fosphorilation-inable-hau-protein-resversible.htmlllllllllllllllllllllllllllllllllllllllllllllllllllllllllllean
This document is subject to copyright. In addition to any fair treatment with the purpose of study or private research, you cannot reproduce any part without written permission. The content is provided only for information purposes.
#Phosphorylation #tau #protein #form #reversible #drops #offering #ideas #early #Alzheimers