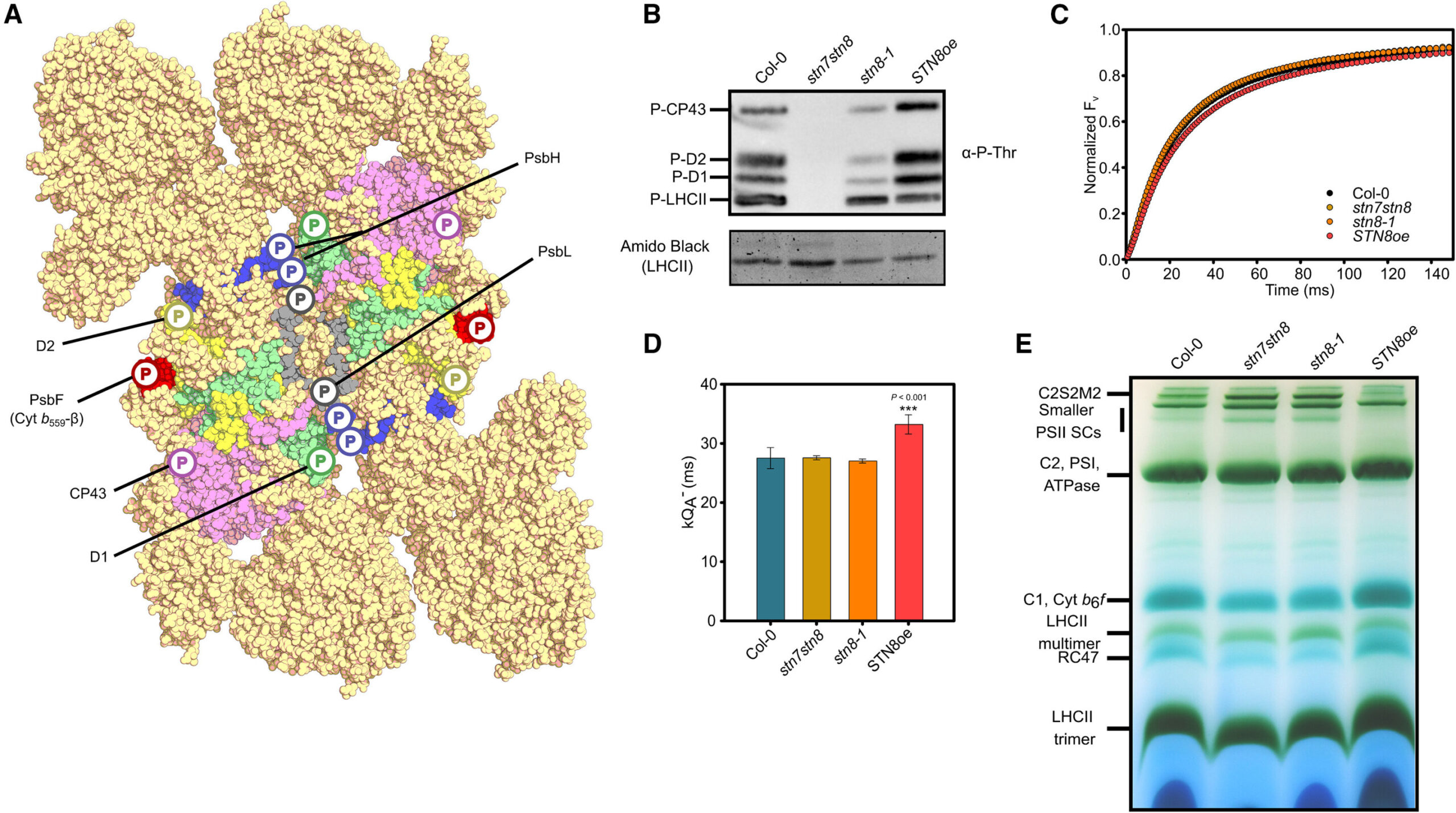
Protein phosphorylation as a promoter of PSII disassembly. Credit: Plant communications (2024). DOI: 10.1016/J.XPLC.2024.101202
Cyanobacteria began to contribute oxygen to the mostly harmful atmosphere of the Earth more than 2 billion years ago. The Photosystem II protein complex now shared by several lineages of cyanobacteria, algae and land plants has served as an important oxygen production site throughout the history of life on earth since then.
Ironically, receiving too much light can damage photosystem II and erode the photosynthetic efficiency of plants. The biochemists of the University of Purdue, Steven McKenzie and Sujith Puthiyaveetil, have obtained new hidden details about how Photosystem II is repaired. The findings of McKenzie and Puthiyaveetil have been published In the newspaper Plant communications.
“Photosystem II divides water and extracts electrons and protons, leaving oxygen as a byproduct. Photosystem II II drives life on Earth,” said Puthiyaveetil, associate professor of biochemistry. Even so, “very little is still understood how these huge protein complexes that use light of light to produce oxygen can be repaired and maintained so efficiently through different lineages of plants, algae and cyanobacteria.”
The long -term objective of the project is to learn to design plants to have better photosynthetic efficiency, said McKenzie, a postdoctoral scholar in biochemistry.
“Photosystem II repair is an energetically expensive process,” said McKenzie, who completed his ph.D. In Purdue in 2024. “You have to disassemble photosystem II, degrade damaged proteins, synthesize new proteins and re -assemble new photo systems. That is strongly expensive for chloroplast.”
The reparation of photosystem II in chloroplasts, the sites of photosynthesis in plants and algae, is already quite efficient, said McKenzie. “But it could make it more efficient when accelerating the repair process or making it less intensive in energy.”
The recent efforts to manipulate the photoprotective pathways of the plant’s photosynthesis have led to greater photosynthetic efficiency in cultivation plants. The genetic engineering of different aspects of the photosystem repair cycle II has the potential for improving photosynthetic efficiency.
Inhibition of the repair cycle can drastically reduce the efficiency of photosynthesis, said Puthiyaveetil.
“This is a key process that is happening all the time. Even with little light, photosystem II is becoming. Damage, especially under a combination of high light and other stressors such as drought, salinity and high temperature.
As photosystem II performs the formidable work of dividing the water using sunlight energy, photodamage suffers. For every 10 million photons, light particles, absorbed by the leaves, a photosystem II is damaged. On a sunny day, a plant leaf intercepts up to 10 quadrillones of photons per second.
The way in which this protein complex is disarmed to eliminate and replace damaged protein to maintain efficient photosynthesis has long persisted as an unresolved question. Photosystem II is huge according to molecular standards, which consists of around 25 protein subunits, some metal centers and chlorophyll scores and other pigment molecules.
The new article shows how the chemical process of adding protein phosphate groups (“protein phosphorylation”) leads some of the disassembly steps of photosystem II in Arabidopsis plants. Scientists have known since 1977 about the phosphorylation of photosystem II. However, what role played in the photosystem II repair cycle had not remained clear.
Purdue scientists originally suspected that phosphorylation was solely responsible for the disassembly of photosystem II. Then, McKenzie suggested that the modification of oxidative protein can also play a role.
“Steve thought that perhaps the oxidative damage of the protein could also be a disassembly mechanism,” Puthiyaveetil said. Additional experimentation revealed that damage to oxidative protein serves as a key mechanism that helps boost photosystem II, especially in the posterior stages. “We were quite surprised by the scope of it. Full credit for Steve.”
Cyanobacteria, red algae and brown, and land plants share the repair mechanism of photosystem II, McKenzie said. What gave him the idea is that cyanobacteria and non -green algae lack phosphorylation of photosystem II and, nevertheless, they can disassemble and repair their photosystems.
“We were interested in knowing if there was an alternative mechanism that could be responsible for promoting the disassembly of photosystem II,” said McKenzie. “That is why we think that perhaps the damage to photosystem II itself could be promoting the disassembly of the complex.”
Phosphorylation seems to serve two functions. “It can boost disassembly, but it could also guarantee a mechanism of quality control for repair,” Puthiyaveetil said. “We say that because once the complex disassembles, the complex must repair.” And plants do not repair their photosystems under persistent light. “They expect high light to disappear. There is some kind of molecular mechanism behind the delay between damage and repairs.”
Then, once the light levels return to normal, the repair and reensamblage of the damaged protein begin. “That is quality control,” said Puthiyaveetil. “Perhaps phosphorylation will avoid the degradation of damaged proteins until they have been paraded since defosphorylation has proven to be a prerequisite for degradation.”
In his experiments, McKenzie used genetically altered plants with variable levels of photosystem phosphorylation II. He also manipulated phosphorylation levels altering the light and source of phosphate groups. In doing so, “we can see what to change phosphorylation levels in photosystem II to the disassembly and repair cycle,” he said.
More information:
Steven D. McKenzie et al, Protein phosphorylation and modification of oxidative proteins promote the restlessness of photos of photos II for repair, repair, Plant communications (2024). DOI: 10.1016/J.XPLC.2024.101202
Citation: Biochemists discover the function of self-repararation in the keysthetic protein (2025, February 10) key complex recovered on February 16, 2025 from https://phys.org/news/2025-02 Biochemists-function-Key -Photosintetic-Protein.html
This document is subject to copyright. In addition to any fair treatment with the purpose of study or private research, you cannot reproduce any part without written permission. The content is provided only for information purposes.
#Biochemists #discover #auto #repair #function #key #photosynthetic #protein #complex