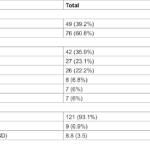
Acute lymphoblastic leukemia (all) is a hematological malignancy that progresses rapidly characterized by the non -controlled proliferation of immature lymphoid progenitor cells, called lymphoblasts, inside the bone marrow and peripheral blood. All can arise from B or T cell lineages, the predominant subtype being B-there. This malignancy represents the most common cancer in children, but also affects adults, where the results remain relatively poorer. The therapeutic landscape of all has evolved drastically in recent decades with advances in chemotherapy, directed agents, immunotherapy and transplantation of hematopoietic stem cells, significantly improving survival rates, especially in pediatric populations.
This article presents a detailed and scientific general vision of all current treatment paradigms, molecularly directed therapies and emerging immunotherapeutic strategies, particularly biespecific antibodies, aimed at scientists, clinical and researchers in hematology-oncology.
1. Pathophysiology and classification of all
Everything is a clonal malignancy that arises from the malignant transformation of lymphoid progenitor cells that do not differ normally. The accumulation of lymphoblasts harms normal hematopoiesis, which leads to the failure of the bone marrow, anemia, thrombocytopenia and immunosuppression. The molecular classification divides everything into subtypes based on lineage (B or T cells) and genetic abnormalities, such as:
-
Philadelphia chromosome (pH+) allcharacterized by the BCR-ABR fusion gene, resulting from T (9; 22) (Q34; Q11) NCI information sheet
-
Mll reorganized everything
-
ETV6-RUNX1 fusion
-
Hyperdiploidia and hypodiploidia
-
Ph-like everythingWith Qinase Activating Alterations
These molecular firms not only guide the prognosis but also an increasingly direct personalized therapy.
2. Treatment General Description: Principles and Phases
The general objective of all treatment is the eradication of leukemic explosions to achieve complete remission (CR), prevent relapse and guarantee long -term disease free survival. Due to the rapid proliferation of lymphoblasts, the treatment must begin immediately after diagnosis.
Therapy is delivered in multiple phases:
2.1 Induction therapy
The main objective of induction is to reduce leukemic load to undetectable levels (<5% of bone marrow explosions), restoring normal hematopoiesis and peripheral blood count. Induction usually lasts 4 to 6 weeks and uses intensive combined chemotherapy regimes.
-
For B-Cell EVERYTHINGstandard induction often includes:
-
Vincristine: A microtubules inhibitor that interrupts mythosis
-
Corticosteroids (prednisone or dexamethasone): Lymphotoxic effects and anti -inflammatory properties
-
Anthracyclines (daunorubicin or doxorubicin): Intercalated DNA, inhibit topoisomerase II
-
Asparaginase: Body extracellular asparagine, crucial for the survival of the leukemic explosion
-
-
For PH+ allInduction incorporates Tyrosine kinase inhibitors (tkis) as Imatinib either Dasatinib aimed at BCR-AB1 IMATINIB APPROVAL.
Patients are hospitalized due to the risk of tumor lysis syndrome, serious cytopenias and infections.
2.2 Consolidation/intensification therapy
After remission, consolidation aims to eliminate minimal residual disease (MRD) and prevent relapse by administration of high chemotherapy combinations or alternatives. The duration is variable but generally implies multiple cycles.
MRD detection due to flow cytometry or therapy intensity PCR guides: detectable MRD patients have a worse prognosis and can receive augmented treatment or consideration for alogenic transplantation of hematopoietic stem cells (allo-HSCT).
2.3 Maintenance therapy
Maintenance therapy extends more than 2-3 years, which generally consists of daily oral 6-Mercaptopurine and weekly MetotrexateWith periodic pulses of Vincristina and Corticosteroids to maintain remission.
Maintenance is crucial to prevent relapse and is less intensive, often based on outpatients.
2.4 Central nervous system prophylaxis (CNS)
The CNS is a common sanctuary site for all. Prophylactic intrathecal chemotherapy (methotrexate, citarabin) is administered routinely during all phases of treatment. You can also use high dose of systemic methotrexate. Cranial irradiation is now reserved for patients with CNS affectation or high risk of relapse to reduce neurotoxicity NCCN guidelines in CNS prophylaxis.
3. Hematopoietic stem cell transplantation
Alogenic stem cell transplant (Allo-HSCT) is considered for high-risk patients, including those with:
-
Persistent MRD after consolidation
-
Adverse cytogenetics (e.g., MLL rearrangements)
-
Early or multiple relapses
Conditioning regimes can be myelolative or reduced intensity. Transplant offers the potential for graft effects versus leukemia, but it entails risks of graft disease against guest and mortality related to treatment Ash Clinical News On HSCT.
4. Therapies directed in all
The directed agents have revolutionized the management of all, especially in refractory or recurring environments.
4.1 Tyrosine kinase inhibitors (TKI)
Tkis aimed at BCR-AB1 (eg, imatinib, dasatinib, Ponatinib) are critical for pH+ all, improving survival when combined with chemotherapy or transplantation NIH Clinical Tests in TKIS.
4.2 Inotouzumab ozogamycin
This conjugate of antibody-herd is directed to CD22, expressed in all B explosions, delivering a cytotoxic useful load (calicheamicin) selectively to leukemic cells. Has shown efficacy in recurrent/refractory b-all FDA Inotouzumab Ozogamycin label.
5. Immunotherapy: BiSpecific Automobile and Antibodies T cells
Immunotherapy is transforming all treatment taking advantage of the patient’s immune system to address selectively leukemic cells.
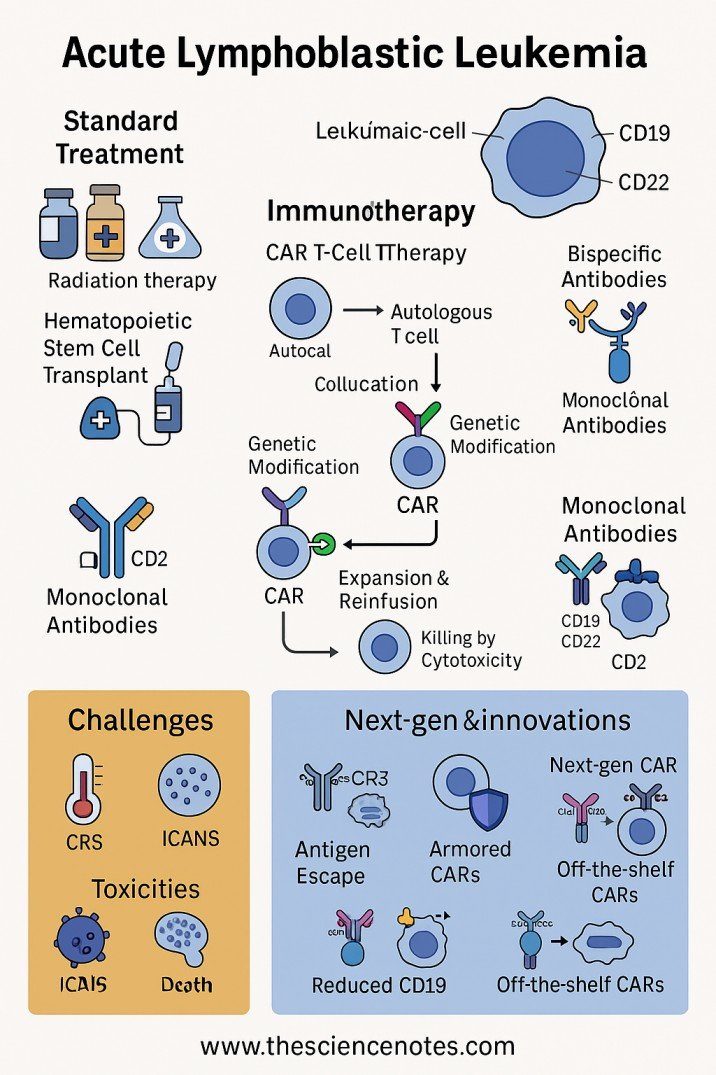
5.1 Cell therapy of chimeric antigen receiver (CAR)
Automobile T cells are genetically designed to express recipients aimed at CD19 in B-Lall explosions, redirecting T cells to recognize and kill leukemia. Car therapies T of CAR approved by the FDA as Tisagenlecluel They have shown remarkable efficacy in recurrent/refractory b-all Approval of the FDA Tisagenlecleceluceluce.
5.2 Biespecific antibodies
Biespecific antibodies (BSAB) represent a new immunotherapeutic class that simultaneously binds two different antigens, typically one on leukemic explosions and another in immune effector cells. This double orientation promotes the formation of a cytolytic synapse, which leads to an effective tumor cell slaughter.
The archetypal BSAB throughout the treatment is Blinatumomab (blincyto):
-
Mechanism: Blinatumomab is a biespecific antibody of T (BIT) cells that binds to CD19 in B and CD3 cell explosions in cytotoxic T lymphocytes (CTL). When physically closing the T cell and tumor cell, it activates T cells regardless of the recognition of greater histocompatibility complex (MHC), which leads to the apoptosis of target cells FDA Blinatumomab label.
-
Clinical use: Blinatumomab is approved by the FDA for all positive MRDs and b-all, a recovered/refractory. It achieves a complete remission in a significant proportion of patients, including those with adverse risk characteristics.
-
Advantages: Blinatumomab offers a non-chemotherapy option that takes advantage of endogenous immune effectors and can serve as a bridge for Allo-HSCT.
-
Limitations: Toxicities such as cytokine liberation syndrome (CRS) and neurotoxicity require monitoring of hospitalized patients, especially during the initial cycles.
-
Ongoing clinical trials: Tests are investigating combinations with chemotherapy or other immunotherapies to improve efficacy and reduce relapse Clinicaltrials.gov blinatumomab studies.
Other biespecific antibodies under investigation include those aimed at CD22 or CD20, which can address the antigen escape seen with therapies aimed at CD19.
6. Recurrent and refractory everything: challenges and innovations
Despite the advances, relapse occurs in 20-30% of adults in all cases and remains the main cause of treatment failure. Every relapse is more resistant, it often requires that rescue regimes are incorporated:
-
Alternative chemotherapy protocols
-
Blinatumomab or Inotouzumab
-
Cell therapy car
-
New agents under clinical investigation (for example, MENINA inhibitors aimed at KMT2A rearrangements) Clinicaltrials.gov Menina inhibitors
Integration of the adaptation of the treatment of MRD monitoring guides.
7. Attention Management and Support Toxicity
Optimal management includes vigilant support to mitigate mylosuppression induced by chemotherapy, infections, tumor lysis syndrome and organ toxicities. This includes antimicrobial prophylaxis, transfusion support, growth factors and management factors.
8. Long -term results and survival
Pediatric All now has healing rates exceeding 80%, a triumph of multidisciplinary treatment. The results of adults are still less favorable, but are improving with the approaches adapted to risk.
Long -term survivors require late effects that include:
The survival programs addressing these problems are vital Survival information of the society of leukemia and lymphoma.
9. Future perspectives in all therapy
The ongoing research aims to refine the molecular diagnosis, deepen the understanding of leukemic biology and develop next -generation therapies, including:
-
Multiple antigen aimed at biespecific antibodies
-
Improved car cell platforms with greater persistence and reduced toxicity
-
Small molecule inhibitors against epigenetic and signage tracks
-
Immune control point block to select all subtypes
-
Multiple multiple of individual cells to characterize leukemic heterogeneity and therapy resistance
-
Microbioma modulation and tumor microambiente orientation
These advances promise further improve cure rates and quality of life.
The treatment of all are incorporated by a complex and multiploque approach that integrates intensive chemotherapy, the therapy aimed at the CNS, the directed agents, immunotherapies that include biespecific antibodies and stem cell transplant. Precision medicine driven by the molecular profile and MRD evaluation allows risk adapted therapies adapted to the patient’s individual biology.
Biespecific antibodies such as Blinatumomab represent a milestone in immune -directed therapies, revolutionizing treatment especially for recurrent or positive disease for MRD. The evolutionary therapeutic panorama promises continuous improvements, driven by translational research and innovative clinical trials, which provides hope of priests in all patient populations.
Additional references and reading:
#Acute #Treatment #Lymphoblastic #Leukemia #TLO #overview #scientific