Normal cells age and eventually die when they have reached their maximum useful life. In these cells, telomeres, the end of a chromosome, cannot be synthesized due to the suppression of the inverse transcriptase enzyme of human telomerase (HTERT), resulting in the shortening of telomeres.
Although the link is not clear yet, many studies showed that HTRT’s reactivation results in the maintenance of telomeres, which could lead to cancer. Meanwhile, it was shown that a couple of telomere union proteins interact with G-Quadruplexes.
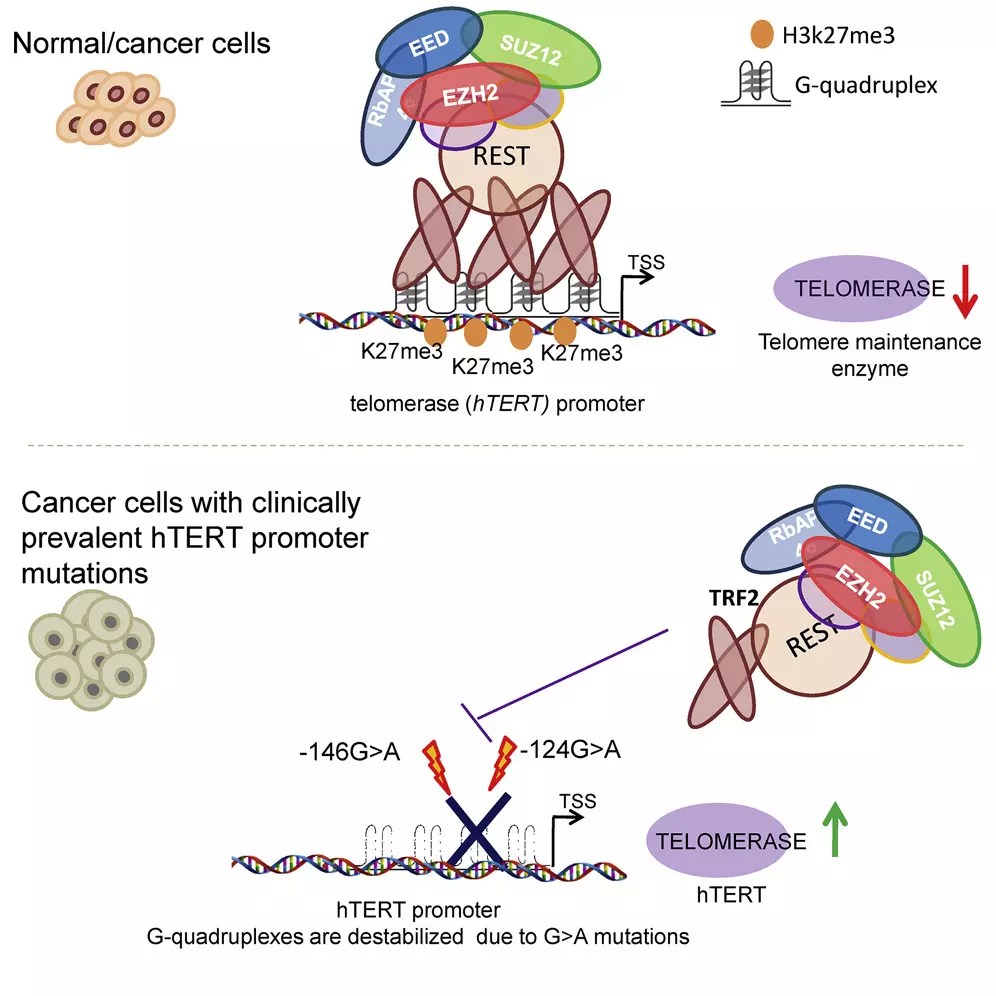
Since the promoter HTRT contains multiple training sequences of G-Cuadruplex, in a recent study, the scientists investigated whether the telomeres 2 (TRF2) repetition factor is associated with the promoter and if this has an effect on the HTERT regulation. Professor Shantanu Chowdhury, Shalu Sharma, Ananda Kishore Mukherjee, Shuvra Shekhar Roy, Sulochana Bagri, Dr. Meenakshi Verma, Antara Senguta, Manish Kumar in the Csir-Institute of Genomic and the integrative biology in collaboration with Dr. Deo Prakash Silje Silje Silje , and the Institute of Genomics and the integrative biology in collaboration with Dr. Deo Prakash Pandey Silje Lije , and Gaute Nesse at the Hospital of the University of Oslo discovered an HTERT regulation mechanism mediated by telomeric factors, establishing molecular links between telomeres and telomerase that could be critical for neoplastic transformation, aging and regenerative therapy that was published in Cellular reports.

The scientists identified the sequence of the promoter Tert through the vertebrates for G-Quadruplex. They also performed a chip test (chromatin immunoprecipitation) and analyzed it. Meanwhile, since TRF2 is a genuine protein of telomere union, the Telomeres PCR was used as a positive control for the TRF2 chip. Later they carry out serial experiments, including protein immunoprecipitation, images with immunofluorescence microscopy to calculate the intensity of the TRF2 and HTRT signal, immuno-fluure citometry, real-time PCR, transfer analysis, ELISA and a pair of other molecular trials.
Professor Chowdhury and his colleagues demonstrated that TRF2 interacts directly with the HTRT promoter and controls Hrtet’s expression and telomerase activity in cancer and normal cells. They also found that both MYB and the primary TRF2 domains are necessary for the transcriptional regulation of HTRT; TRF2 regulates the epigenetic state of chromatin in the promoter HTRT; Recruitment induced by TRF2 of the Polycomb Representative Complex (PRC2) to the promoter HTRT.
The team discovered that the TRF2 association with the promoter HTRT does not depend on telomeres, but depends on G-Quadruplex. In addition, in cancers with HTRT promoting mutations, the occupation of TRF2 is lost and when stabilizing the G-Quadruplex with ligands, the TRF2 union is restored and the activated telomerase is suppressed.
Their findings will help to understand more the processes of response to aging, cancer, cell senescence and DNA damage related to the maintenance of telomeres and telomerase regulation. The main author, Professor Chowdhury said: “The case of telomeres in cancer is particularly relevant. Although more work will be required to prove this, based on our findings here, the establishment of the diaphony of telomeres-solerase through TRF2 (telomeric together with the non-television union in the promoter HTRT) can be key to how the Telomeres in cancer cells. “
He also added: “Relatively improved telomerase levels and long telomeres are crucial for the maintenance/survival of pluripotent stem cells.” Therefore, the HTRT regulation mediated by TRF2, linked to telomeres, could be significant in pluripotence.
This study that shows for the first time that HTRT TRF2 -induced reapp Sanity in multiform glioblastoma and other types of cancer that use small molecules ligand offers a potential therapeutic opportunity. Small molecules adapted to bind and stabilize the Cuplex G. “These results present interesting opportunities to develop g-quadruplex binding molecules for therapeutic intervention in multiform glioblastoma, devastating brain cancer,” Professor Chowdhury observed. In addition, the mechanisms that HTRT maintain in a repressed state in normal cells and the deregulation of which resulted in HTERT reactivation in cancer cells have been demonstrated.
“Together, these results suggest molecular links between telomeres and telomerase that could be critical to advance the understanding of cellular intrinastic functions, including neoplastic transformation, aging and pluripotence/differentiation,” said Professor Chowdhury.

Newspaper reference:
Sharma S, Mukherjee Ak, Roy SS, Bagri S, Lier S, Verma M, Senguta A, Kumar M, Nesse G, Pandey DP, Chowdhury S. Human telomerase is directly regulated by the interaction TRF2-G-Quadruplex non-telomeric. Cellular rep. 2021 May 18; 35 (7): 109154. DOI: 10.1016/J. CELREP.2021.109154. PMID: 34010660.
About the authors

Dr. Shalu Sharma, Ph.D.
After completing my Master in Microbiology, in 2014, Dr. Shalu Sharma joined Dr. Shantanu Chowdhury in Csir-Igib for PHD. SC Lab Study cancer with focus on the biology of telomeres. He began exploring the regulatory effects of telomeres on cell and transcriptomic aging of the entire genome. Little by little, he was interested in understanding the regulation of human telomerase, hyperactivated in> 90% of cancers. The recent findings of the research team reveal how telomeres could indicate the regulation of telomerase. How clinical mutations in the Telomerase promoter cause telomerase hyperactivation. Based on this, the team found the potential of small DNA binding molecules for therapeutic interventions.

Dr. Ananda K Mukherjee, PhD
Dr. Ananda K Mukherjee joined as a postgraduate student in the laboratory of Dr. Shantanu Chowdhury in IGIB in 2014, where she was presented to the biology of the telomeres and the protein of union to the telomeres-TRF2. The team made an interesting observation that the non -canonical transcriptional effects of TRF2 depended on the length of the telomeres. Subsequently, they noticed that TRF2 directly represses the telomerase by keeping the histones suppressors using G-Quadruplexes in the gene promoter. In addition, they discovered that specific glioblastoma mutations that interrupt G-Quadruplex led to the loss of this regulation. He is currently trying to understand how the heterogeneity of the telomeres of tumor cells could influence the immune response to cancer.

Professor Shantanu Chowdhury, Ph.D.
Shantanu Chowdhury is currently a professor at the CSIR-Institute of Genomics and Integrative Biology in Delhi. Their research interests include understanding the function of non-duplex DNA structures called G-Quadruplexes. Particularly, in the control of telomerase and the function of telomeres in cancer. In 2002 he moved to his current position where his own laboratory began. In 2012, Shantanu received the Shanti Swarup Bhatnagar Prize in Biological Sciences (most prestigious scientific award in India). He is a senior member of the DBT / Wellcoma Trust India Alliance and is a member of the Editorial Board of the Journal of Biological Chemistry.
Main image credit: Chowdhury et al https://doi.org/10.1016/j.cerep.2021.109154
#Gquadruplexes #reveal #molecular #links #telomeres #telomerase #critical #findings #neoplastic #transformation #aging #regenerative #therapy