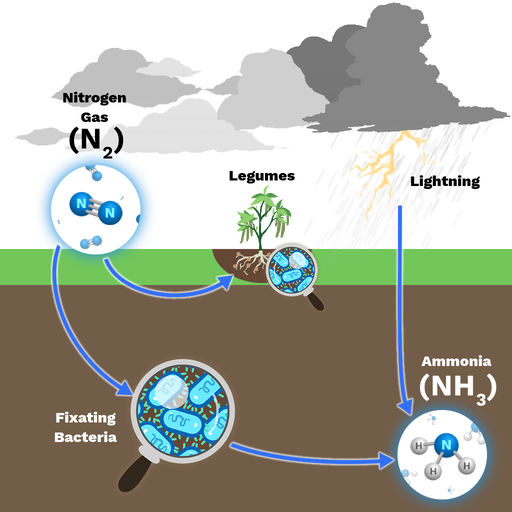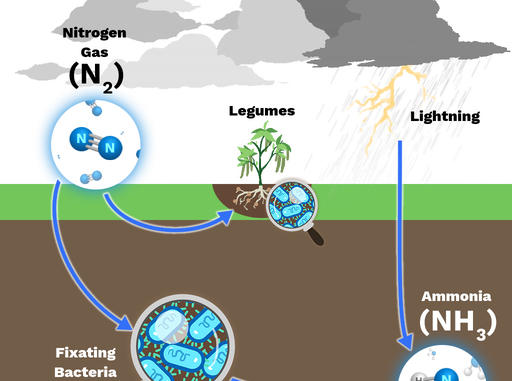
What is nitrogen fixation?
- The phenomenon of free nitrogen conversion into nitrogen salts so that it is available for plants absorption is called nitrogen fixation.
- On the basis of the agents through which nitrogen is set, nitrogen fixation is divided into two types:
- Physical nitrogen fixation
- Biological nitrogen fixation
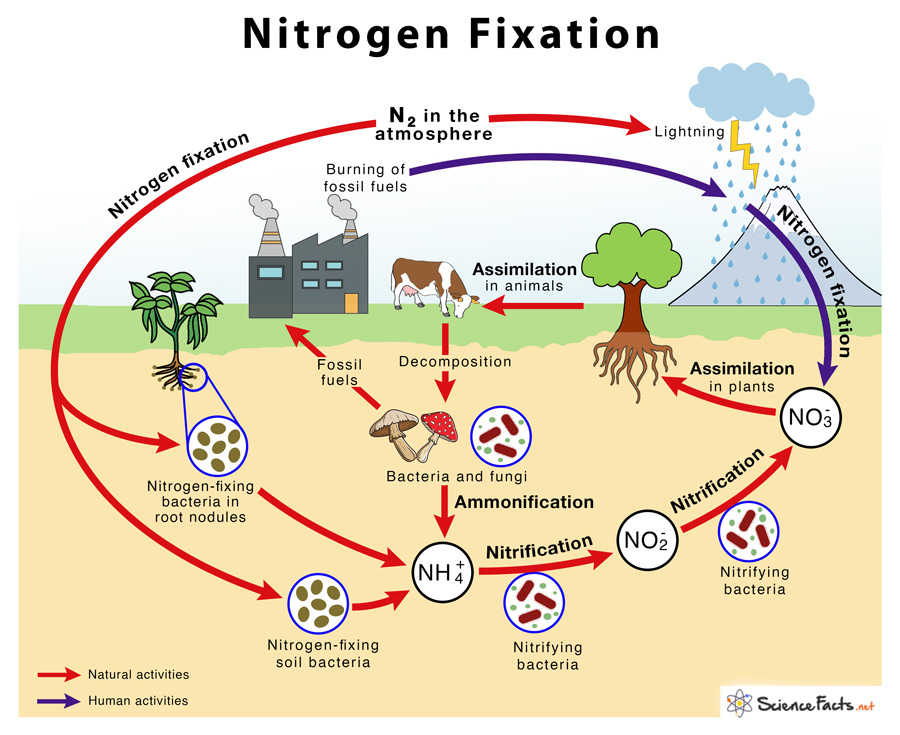
Physical nitrogen fixation:
- Of the total nitrogen set by natural agencies, approximately 10% of this occurs due to physical processes such as rays (that is, electric shock), thunder storms and air pollution.
- Ray and UV radiation in the atmosphere favor the combination of gaseous nitrogen and oxygen to form nitric oxide (NO). Nitric oxides oxidize again with oxygen to form nitrogen peroxide (NO2).
north2+O2 ————> 2no (during ray and the storm)
2no+o2 ————> 22 (Oxidation)
- This nitrogen peroxide can be combined with water during rains to form nitrous acid and nitric acid. Acids fall on the ground along with rainwater and react with alkaline radicals to produce water soluble nitrates (no3–) and nitrites (no2–).
22 + H2Or ——> hno2 + Hno3
HNO3 + Ca ok salts —————-> ca ok nitrates
- Nitrates are water soluble and are directly absorbed by plants.
Biological nitrogen fixation:
- Atmospheric nitrogen conversion (molecular nitrogen) in inorganic or organic ways with the help of living organisms is called biological nitrogen fixation.
- This process is transported by two main types of microorganisms;
- Those who are free or asympbiotics
- And those who live in a close symbiotic association with other plants
- However, a third category of microbes that set nitrogen in association with cereal and pastures roots have been recognized. It is called associative symbiotic nitrogen.

Asymptbiotic nitrogen fixation:
- A large number of free free bacteria and cyanobacteria set atmospheric nitrogen in a usably form.
- These microorganisms can be classified as follows:
- Aerobic free life nitrogen fixing bacteria. e.g PHYETOBACTER, BEIJERINKIA, DERXIA etc
- Anaerobic free -life nitrogen fixing bacteria. e.g Clostridium pasteurianum, Bacillus etc
- Nitrogen fixing bacteria photo-autopotrophic free life. e.g Clorobium, Rhodopseudomonas, Rodospirellum etc
- Chemosynthetic Nitrogen Fixing Bacteria of Free Life. e.g Desulfovibrio
- Free nitrogen fixing cyanobacteria: It is known that a large number of heterocist cyanobacteria or blue green algae set atmospheric nitrogen. e.g Nostoc, Anabaena, Cylindrospermum, Trichodesmium, Calothrix etc Some of free living cyanobacteria fix nitrogen in rice fields (for example Aulosira fertilissima), some set the nitrogen actively in sugar and corn cane fields (for example, Cylindron)
Symbiotic biological nitrogen fixation:
- Many bacteria and cyanobacteria set atmospheric nitrogen in symbiotic association with other plants. Some common examples are:
- Rizobium Legosarum (Bacteria) in association with legumin plant roots.
- Nostoc (Cyanobacteria) in association with a briophite (Antocero).
- Anabaena Azolola (Cyanobacteria) in association with a water fern (Azolla).
- Anabaena and Nostoc In association with the coralloid roots of a gymnosperms, cycas.
Symbiotic nitrogen fixation in legume plants:
- One of the bacteria that sets the most important nitrogen, Rizobio Nitrogen correction in symbiotic association with roots of legume plants such as peas, beans, clover, alpha alpha, lipinas, etc.
1. Chemical interaction and entrance:
- Initially, bacteria grow on the ground near the roots of the upper plants, where they do not fix nitrogen. When these bacteria come into contact with the roots of legume plants, they interact chemically and enter the roots through the root hairs.
- The entrance of bacteria in the root hair depends on several chemical signals sent from the root hair.
2. Infection thread formation:
- The enzymes produced by bacteria degrade the parts of the cell wall of the root hair that produces a thread as the structure called infection thread.
- These bacteria multiply and invade the infection thread and finally reach the internal cortex where they enter tetraploid cells and stimulate them to divide.
3. Root and bacteroid nodule formation:
- The proliferating cells form a protuberance similar to the knob called root nodule. These bacteria multiply and colonize inside the tetraploid cells until the available cytosol is filled after which they become inactive.
- Each non -mobile and latent bacteria is called Bactereroid. A typical root nodule cell contains several thousand bacterteroids.
4. Peribaceroid membrane and peribaceroid space:
- In general, bacteroids occur within the cytoplasm in groups. Each group is surrounded by a membrane called Peribaceroid membrane. The space inside the cell surrounded by peribaceroid membrane is called Peribaceroid space.
- A red pigment called Leghemoglobin It is filled out of the Peribacterial space in the cytosol of the nodule cells.
- Leghaemoglobin is similar to hemoglobin in our red blood cells, and has the ability to combine very rapidly with oxygen and, therefore, acts like a very efficient O2 scavenger.
See also nitrogen Cycle
Biological nitrogen fixing mechanism:
- There are some basic nitrogen fixation requirements;
- Presence of nitrogenase and hydrogenase enzyme
- A protection mechanism for the nitrogenase enzyme against or2
- An iron protein that is not Hema, Ferredoxin as an electron bearer
- System that contains hydrogen such as pyruvate, hydrogen, sucrose, glucose, etc.
- A constant ATP supply
- Presence of thiamine pyrophosphate (TPP), Coenzyme-A, inorganic phosphate and mg2+ as co-factors
- Presence of cobalt and molybdenum
- A carbon compound for ammonia released entrapment
- In the process of fixing biological nitrogen due to free life and symbiotic nitrogen fixers, the nitrogen molecule is progressively reduced step by step to the ammonia by adding hydrogen atom pairs.
- Pyruvic acid serves as an electron donor (hydrogen donor) in most cases. However, it has also been shown that other electron donation systems such as hydrogen, sucrose, glucose, etc. They work in different systems.
- In legume plants, the glucose-6-phosphate molecule probably acts as a substrate to donate hydrogen.
- The general process occurs in the presence of enzyme, nitrogenase, which is active in the anaerobic condition.
- Nitrogenase consists of two subunits;
- an iron protein does not commonly called faith protein also called Dentrogen reductase.
- and an iron-molibdeno protein called Mo-fe protein or Dinitrogenase
- Both subunits are necessary for enzyme activity. This faith protein component reacts with ATP and reduces Mo-fe protein, which then reduces nitrogen to ammonia.
- Oevell’s biochemical reaction is as follows:
north2 + 8e– + 8 h+ + + 16 ATP ———-> 2NH3 + H2 + 16 ADP + 16 Pi
- The product of nitrogen fixing is the ammonia that is toxic to plants. However, ammonium ions are safely assimilated by the upper plants.
- In most cases, ammonium ions are transformed into amino acids and then translocate ‘
Ammonia + ∝-cetoglutarate + nadh ——-> glutamate + nad + + h2O (dehydrogenase action)
- In the case of legumes, the ammonia is absorbed by the host and is immediately assimilated organically (that is, amino acids, amides or ureides) and then translocates through vascular tissues to other parts of the plant.
- Most of the ammonia produced from free nitrogen fixers is directly metabolized by microorganisms, absorbed by the upper plants or converted to nitrates by the process of nitrification.
Nitrification:
- The ammonia oxidizes nitrite ions and nitrate by a group of nitrifying bacteria that inhabit the ground. For example, nitrosomonas and nitrobacter.
- These nitrifying bacteria obtain their energy from the oxidation of ammonium and nitrite ions.
- The Nitrosomonas group oxidizes the ammonia to the nitrites;
N.H4+ + + 3/2 o2 <———–> No2– + H2Or + h+ + -84kcal ( By Nitrosomonas)
- The Nitrobacter group oxida nitrites to nitrates;
NO2– + 1/2 o2 ———> no3– + -17.8 Kcal (By Nitrobacero)
- Nitrates (no3–), produced by nitrification, are absorbed by higher plants and assimilated by the process called Nitrate assimilation.
Assimilation of nitrate in plants:
- The nitrate reduction process in ammonia is called nitrogen assimilation or nitrate assimilation.
- The absorption of nitrate by plant roots from the soil is a process mediated, active and energy dependent.
- Nitrates absorbed by the roots of the plant become amino acids and amidas before joining in proteins and other macromolecules.
- In most plants, nitrate reduction occurs in root tissues and then transported to shoot through the xylem, while in others, the process occurs in leaves and stems. The general summary equation of the reduction of nitrate to ammonia is as follows:
NO3– + 8e– + 10h+ + ——–> NH4+ + + 3h2EITHER
- This reduction process consists of the next two different enzymatic steps:
- The first step is the conversion of nitrate to nitrito. The reaction is catalyzed by the enzyme reductase nitrate (sulfhydryl containing Molybdoflavhemoprotein). This step occurs in the cytosol outside any organelle and requires NADH (or in a few NADPH species) as a donor of electrons, FAD as a prosthetic group, cytochrome B557 as an electron bearer and molybdenum (MO) as an enzyme activator. The transfer of electrons of the road in the reduction of nitrate to nitrito is as follows:
NADH + H.+ + + Fad ——–> Nad+ + + Fadh2
(or Nadph+ + + H+ +) (or NADP+ +)
FADH2 + (Oxi) mo ——> (red) mo + 2h+ +
(Red) mo + 2h+ + + No3 ——–> (oxi) mo + no2
-
- The second step is the reduction of nitrite to the ammonium ion. The reaction is catalyzed by the enzyme Nitrite reductase. The most likely electrons donor in this reaction seems to be reduced from ferredoxin (a non -heme protein that contains lower molecular weight). Ferredoxin is reduced in the light reaction of photosynthesis in green leaves. The nature of the electron donor in the non -green tissue (eg, roots) is unknown. In non -green tissues, some unknown electron carriers (probably FADH2 or NADPH + H+ +), generated in the respiratory metabolism dona electrons. The general reaction of nitrite reduction is as follows:
NO2 + 6e– + 8h+ + ——–> NH4+ + + 2h2EITHER
Nitrogen fixation, their types and mechanism
#Nitrogen #fixation #types #mechanism