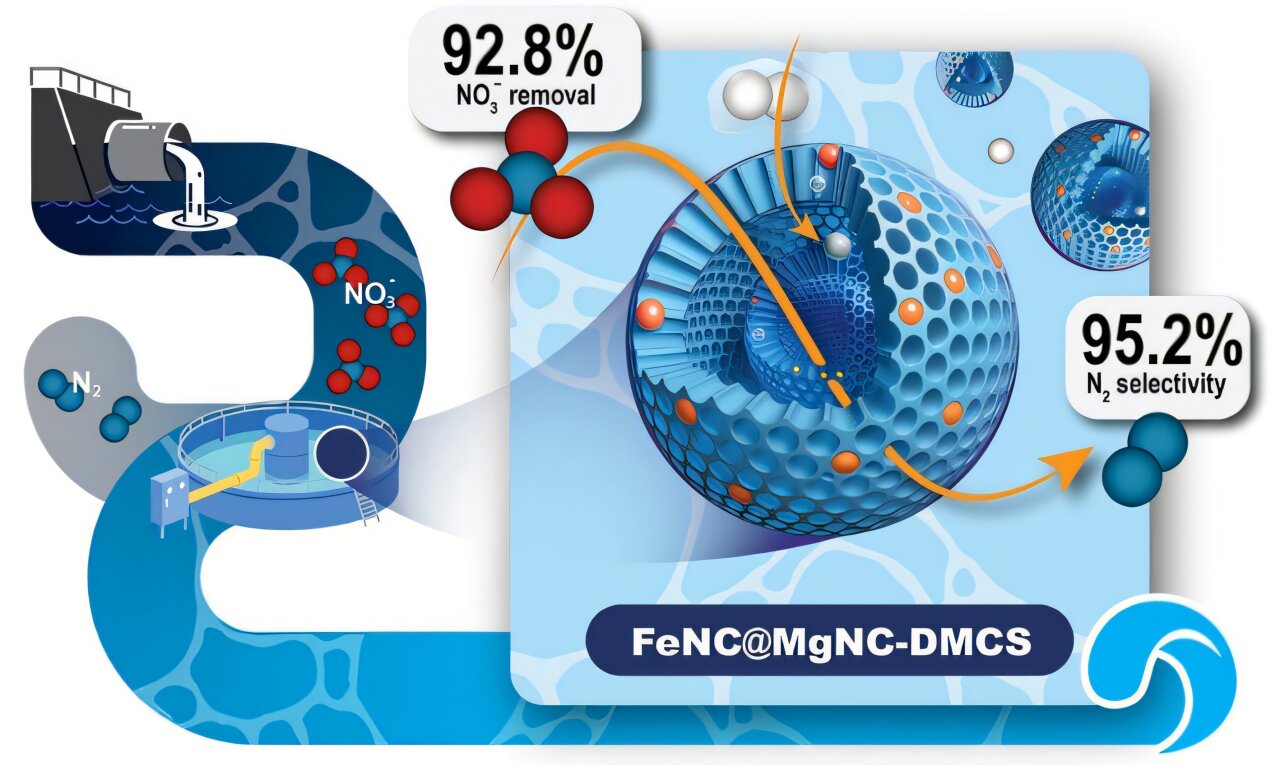
Illustration of double layer mesoporous carbon sphere catalyst with single atom dual sites. The inner Fe-N₄ centers drive nitrogen-nitrogen coupling, while the outer Mg-N₄ shell acts as a proton barrier, directing the reaction toward harmless nitrogen gas (N₂) instead of ammonia. This design achieves high nitrate removal and exceptional selectivity, offering a sustainable solution for clean water treatment. Credit: Eco-Environment and Health
Nitrate pollution in water threatens ecosystems and human health, but efficiently removing them without producing harmful byproducts remains a challenge. A new study reports a monatomic dual catalyst engineered into double-layered mesoporous carbon spheres that achieves high activity and selectivity.
Excessive nitrate levels in groundwater and wastewater often come from agriculture, wastewater, and industrial effluents, leading to eutrophication, ecological imbalance, and health risks such as methemoglobinemia. Traditional treatment methods, including biological denitrification, membrane separation, and adsorption, suffer from high costs, energy demands, or secondary pollution.
Electrocatalytic denitrification has emerged as an attractive alternative, directly converting nitrate to ammonia or nitrogen gas. However, most catalysts favor ammonia formation due to easier hydrogenation pathways, raising issues of toxicity and recovery costs. Considering these challenges, there is an urgent need to design catalysts that selectively convert nitrate into harmless nitrogen gas, ensuring sustainable water treatment.
Researchers at Jiangnan University have developed a novel dual-monatomic catalyst that selectively converts nitrate to nitrogen gas with exceptional efficiency. The study, published in Eco-environment and healthdemonstrates how double-layered mesoporous carbon spheres hosting iron and magnesium atomic sites enable almost complete removal of nitrates while preventing harmful ammonia production. With a nitrate conversion of 92.8% and a nitrogen selectivity of 95.2%, the catalyst showed remarkable stability in long-term flow cell operation, highlighting its potential to advance sustainable wastewater treatment technologies.
The team designed the FeNC@MgNC-DMCS catalyst using a sequential modular assembly and pyrolysis strategy, producing double-layer mesoporous carbon spheres with spatially separated atomic sites. The inner layer contains Fe-N.4 sites that accelerate nitrogen-nitrogen coupling, while the outer Mg-N4 The layer creates a moderate basicity, acting as a “proton fence” to regulate hydrogen distribution. This architecture minimizes competitive hydrogenation that would otherwise produce ammonia.
Laboratory testing revealed that the optimized catalyst achieved 92.8% nitrate removal with 95.2% nitrogen selectivity, far outperforming single-shell or single-metal controls. Mechanistic studies using in situ mass spectrometry and infrared spectroscopy confirmed that the reaction pathway favored N–N coupling rather than N–H hydrogenation.
The catalyst also demonstrated resilience over a wide range of pH and varying nitrate concentrations, while maintaining high selectivity in the presence of interfering ions. In continuous flow cell experiments with simulated wastewater, the catalyst retained >90% removal and >93% nitrogen selectivity for 250 hours. Importantly, Fe and Mg leaching was minimal and well below World Health Organization drinking water standards, underscoring its structural stability and environmental safety.
“This work illustrates how careful atomic engineering can fundamentally change reaction pathways in electrocatalysis,” said Professor Hua Zou, co-corresponding author of the study. “By introducing a magnesium-based proton barrier around the iron catalytic centers, we effectively prevented the secondary reactions that lead to the formation of ammonia. The result is a catalyst that not only achieves high activity but also unprecedented nitrogen selectivity. These advances pave the way towards practical and scalable solutions for nitrate pollution, which is a pressing issue for the global sustainability of the water”.
The development of FeNC@MgNC-DMCS catalysts opens new possibilities for clean water technologies. With its high nitrate removal efficiency, excellent nitrogen selectivity and long-term durability, the system is particularly suitable for wastewater treatment in agricultural and industrial environments where nitrate pollution is severe.
Beyond water purification, the design strategy (which combines single-atom dual sites within a hierarchical carbon framework) provides a blueprint for adapting other catalytic processes that require balancing competing reaction pathways. By addressing both environmental safety and operational feasibility, this work contributes to global efforts aimed at mitigating nitrate pollution and promoting sustainable resource management.
More information:
Wanchao Song et al, N-selective electrocatalytic denitrification2 through single-atom dual sites in double-layered mesoporous carbon spheres, Eco-environment and health (2025). DOI: 10.1016/j.eehl.2025.100172
Provided by Nanjing Institute of Environmental Sciences
Citation: Double-shelled carbon spheres drive cleaner conversion of nitrate to nitrogen (2025, October 17) retrieved October 25, 2025 from https://phys.org/news/2025-10-shelled-carbon-spheres-cleaner-nitrate.html
This document is subject to copyright. Apart from any fair dealing for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.
#Doublelayer #carbon #spheres #drive #cleaner #conversion #nitrate #nitrogen