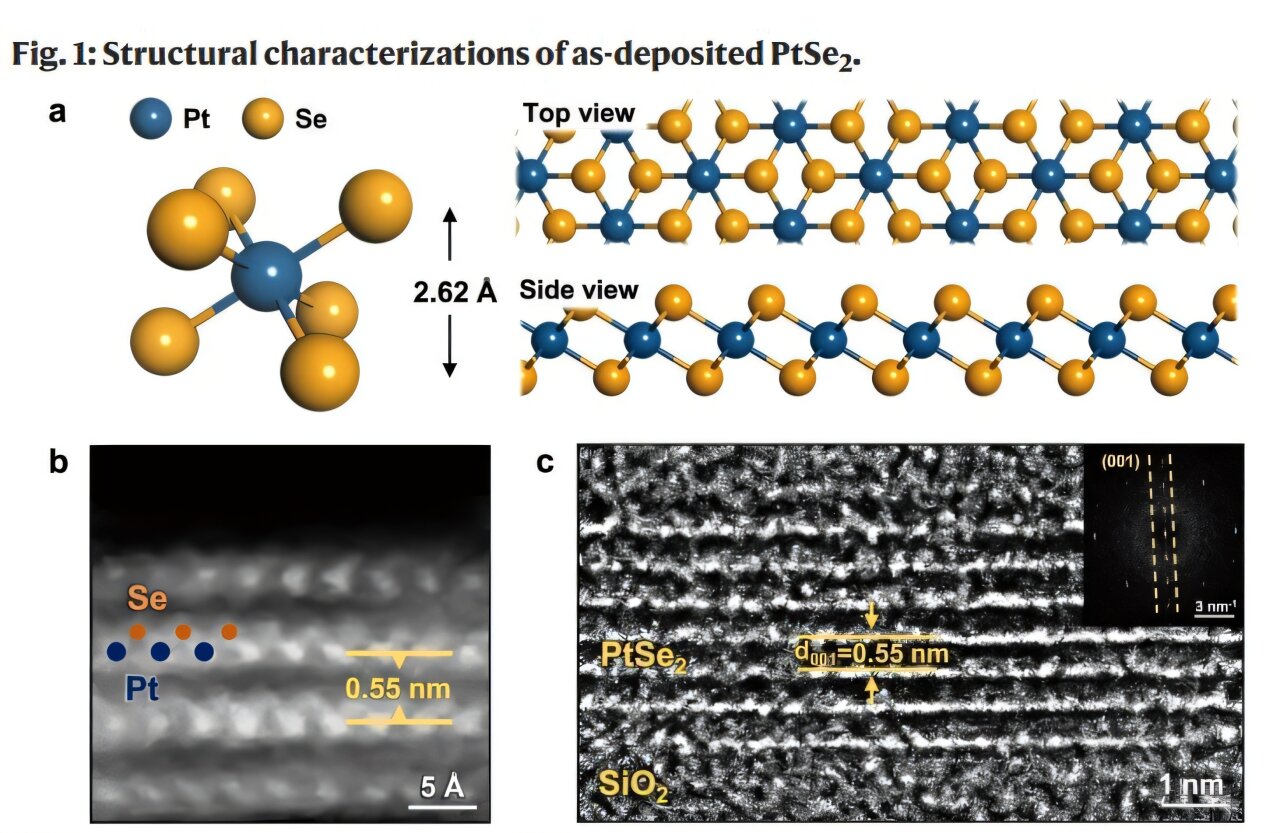
Credit: Nature communications (2025). DOI: 10.1038/S41467-025-61320-0
Platinum Selenaid is a two -dimensional material formed by the combination in platinum layers (PT) and Selenium (SE). Its excellent crystallinity and precise control of the interactions between layers allow the modulation of several physical and chemical properties. It has been actively investigated in several fields, such as semiconductors, photodetector and electrochemical devices.
Now, a research team has proposed a new design concept where platinum at the atomic level present on the platinum selenaid surface can function as a catalyst for gas reactions. Through this, they have demonstrated their potential as next -generation gas phase catalyst technology for high efficiency carbon dioxide conversion and carbon monoxide reduction.
A Joint Research Team Led By Endowed Chair Professor Jeong Young Park from The Department of Chemistry, Along With Professor Hyun You Kim’s Team From Chungnam National University and Professor Yeonwoong Jung’s Team From The University of Central Florid MONOXIDE OXIDATION PERFORMANCE BY USING PLATINUM ATOMS EXPOSED ON THE SURFACE OF THE TWO-DIMENSIONAL TRANSITION METAL DICHALCOGENID, PLATINUM DISELENIDE (PTSE₂). Their findings are published in Nature communications.
To maximize catalytic performance, the research team designed the catalyst so that platinum atoms are highly scattered on the surface, moving away from conventional forms of bulk platinum catalyst. This approach allows more catalytic reactions with a lower amount of platinum and promotes active electronic interaction between platinum and selenium by controlling the electronic surface structure.
The thin film of platinum deelenur, several thick nanometers, showed a higher carbon monoxide oxidation performance throughout the temperature range compared to a thin platinum general film under the same conditions.
In particular, on the surface, carbon monoxide and oxygen are adsorbed uniformly in similar proportions, which increased the opportunity to react with each other, which significantly improved the catalytic reaction. The key to this performance improvement lies in the largest exposure of surface platinum atoms due to “selenium vacancies (SE)”, which also increased adsorption sites for gases.
The research team confirmed in real time that these platinum atoms acted as adsorption sites during the actual reaction process through the spectroscopy analysis of X-ray photoelectrons of ambient pressure (AP-XPS) conducted in the Pohang accelerators laboratory. This high precision analysis was possible by advanced equipment capable of observing surfaces at the level of 1 nanometer in an environmental pressure environment. Simultaneously, computer simulation calculations (functional density theory) theoretically demonstrated that platinum desenurus has different electron flow characteristics than general platinum.
Professor Jeong Young Park declared: “This research presents a new design strategy that uses platinum desenurus, a different two -dimensional layer structure from conventional platinum catalysts, to cause specialized catalytic functions for gas reactions.
“The electronic interaction between platinum and selenium created reaction conditions for the balanced adsorption of carbon monoxide and oxygen, and when designing it to have a higher reactivity in the entire temperature range than conventional platinum, its practical applicability has improved. This allowed us Adsorption and high efficiency adsorption, “” “. “
This research was co -author of Dr. Gyuho Han of Kaist’s Department of Chemistry, Dr. Hyuk Choi of the Department of Science and Materials Engineering of the National University of Chungnam, and Professor Jong Hun Kim of the University of Inha.
More information:
Gyuho Han et al, improved catalytic activity in PTSE atomically dispersed2 two -dimensional layers, Nature communications (2025). DOI: 10.1038/S41467-025-61320-0
Citation: The atomic level platinum catalyst increases the elimination of air carbon monoxide (2025, July 22) recovered on July 25, 2025 from https://phys.org/news/2025-07-atomic-platinum-catalyst-boosts-carbon.html
This document is subject to copyright. In addition to any fair treatment with the purpose of study or private research, you cannot reproduce any part without written permission. The content is provided only for information purposes.
#Atomic #level #platinum #catalyst #increases #elimination #air #carbon #monoxide